-
Name
2-Acetylbenzo[b]thiophene
- EINECS 245-177-7
- CAS No. 22720-75-8
- Article Data37
- CAS DataBase
- Density 1.219 g/cm3
- Solubility
- Melting Point 86-88 ºC
- Formula C10H8OS
- Boiling Point 304.5 ºC at 760 mmHg
- Molecular Weight 176.239
- Flash Point 138 ºC
- Transport Information
- Appearance white to light yellow crystal powder
- Safety 36
- Risk Codes 20/21/22-22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Ketone,benzo[b]thien-2-yl methyl (7CI,8CI);1-(Benzo[b]thiophen-2-yl)ethan-1-one;1-Benzo[b]thiophen-2-ylethanone;2-Acetylbenzothiophene;
- PSA 45.31000
- LogP 3.10390
Synthetic route
-
-
53606-33-0
2-(ethylsulfanyl)benzenecarbaldehyde

-
-
78-95-5
chloroacetone

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| With calcium oxide In acetone at 59℃; | 100% |
| With calcium oxide for 3h; Reflux; |
-
-
51868-95-2
1-(benzo[b]thiophen-2-yl)ethanol

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| With [bis({2‐[bis(propan‐2‐yl)phosphanyl]ethyl})amine](borohydride)(carbonyl)(hydride)iron(II); potassium tert-butylate; acetone In n-heptane at 50℃; for 24h; Schlenk technique; Inert atmosphere; | 98% |
| With chromium(VI) oxide; sulfuric acid; water In acetone at 20℃; for 24h; Inert atmosphere; | 94% |
| With pyridinium chlorochromate In dichloromethane at 20℃; for 10h; | 78% |

| Conditions | Yield |
|---|---|
| In diethyl ether; water | 95% |

-
-
7022-45-9
2-(methylsulfanyl)benzaldehyde

-
-
78-95-5
chloroacetone

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| With magnesium oxide In toluene at 120℃; for 4h; Reagent/catalyst; | 95% |
-
-
55164-16-4
2,2'-dithiodibenzaldehyde

-
-
78-95-5
chloroacetone

-
-
123-54-6
acetylacetone

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| Stage #1: 2,2'-dithiodibenzaldehyde; acetylacetone With potassium carbonate; dimethyl sulfoxide at 20℃; for 1.5h; Inert atmosphere; Stage #2: chloroacetone at 50℃; for 1.5h; Inert atmosphere; | 94% |

-
-
148520-10-9
IMB-05

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| With ammonium chloride; zinc In ethanol at 0℃; for 1h; | 93% |
-
-
89-98-5
2-chloro-benzaldehyde

-
-
24653-75-6
1-mercaptopropan-2-one

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| With potassium carbonate In water at 90℃; for 2h; Green chemistry; | 84% |

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| With copper diacetate; potassium ethyl xanthogenate In dimethyl sulfoxide at 100℃; for 3h; | 84% |
-
-
135555-29-2
2-(prop-1-en-2-yl)benzo[b]thiophene

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| With 1,1'-bis-(diphenylphosphino)ferrocene; oxygen; 2,5-Dimercapto-1,3,4-thiadiazole In acetonitrile at 20 - 80℃; under 760.051 Torr; for 15h; Schlenk technique; | 82% |
-
-
130966-65-3
2-(hydroxymethyl)thiophenoxyacetone

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| With pyridine-SO3 complex; triethylamine In dichloromethane; dimethyl sulfoxide | 79% |

| Conditions | Yield |
|---|---|
| Stage #1: Benzo[b]thiophene With n-butyllithium In diethyl ether; hexane at -78℃; for 1.5h; Reflux; Stage #2: N,N-dimethyl acetamide In diethyl ether; hexane for 2h; Reflux; | 78% |
| With n-butyllithium In tetrahydrofuran; hexane at -78 - 22℃; | 35% |
-
-
7022-45-9
2-(methylsulfanyl)benzaldehyde

-
-
78-95-5
chloroacetone

-
A
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| With magnesium hydroxide In neat (no solvent) at 120℃; for 3h; | A 71% B 22.5 %Chromat. |

-
-
122752-74-3
(E)-4-(2-iodophenyl)but-3-en-2-one

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium sulfide In N,N-dimethyl-formamide at 130℃; for 12h; Schlenk technique; Sealed tube; | 65% |
-
-
78191-00-1
N-Methoxy-N-methylacetamide

-
-
95-15-8
Benzo[b]thiophene

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane at -78 - 22℃; | 62% |
| With n-butyllithium In tetrahydrofuran; ethanol; pentane |
-
-
1196-81-2
2-ethylbenzo[b]thiophene

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite; N-hydroxyphthalimide; oxygen In benzonitrile at 80℃; under 760.051 Torr; for 24h; Schlenk technique; | 60% |
-
-
55164-16-4
2,2'-dithiodibenzaldehyde

-
-
123-54-6
acetylacetone

-
A
-
22720-75-8
2-acetyl benzo[b]thiophene

-
B
-
1005199-10-9
2-mercaptobenzaldehyde potassium salt

| Conditions | Yield |
|---|---|
| With potassium carbonate; dimethyl sulfoxide | A 45% B n/a |


| Conditions | Yield |
|---|---|
| Stage #1: benzaldehyde With sulfur; n-butyllithium; N,N',N'-trimethylenediamine In tetrahydrofuran; hexane at -20℃; for 3h; Dehydrogenation; substitution; Stage #2: chloroacetone With potassium carbonate In tetrahydrofuran for 24h; Alkylation; cyclization; | 38% |

-
-
1423-60-5
methyl ethynyl ketone

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| With copper In acetone Ambient temperature; | 37% |
-
-
50779-55-0
benzothiophen-2-yllithium

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| With lithium acetate |
-
-
108-24-7
acetic anhydride

-
-
95-15-8
Benzo[b]thiophene

-
A
-
22720-75-8
2-acetyl benzo[b]thiophene

-
B
-
1128-05-8
3-acetylbenzothiophene

| Conditions | Yield |
|---|---|
| With boron fluoride ether | |
| With trifluoroacetic acid at 20℃; for 1.5h; Friedel-Crafts Acylation; Overall yield = 92 %; Overall yield = 121 mg; |

-
-
108-98-5
thiophenol

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
78-95-5
chloroacetone

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction; |

-
-
75-36-5
acetyl chloride

-
-
95-15-8
Benzo[b]thiophene

-
A
-
22720-75-8
2-acetyl benzo[b]thiophene

-
B
-
1128-05-8
3-acetylbenzothiophene

| Conditions | Yield |
|---|---|
| With aluminium trichloride In 1,2-dichloro-ethane Ambient temperature; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| Stage #1: acetyl chloride With aluminium trichloride In nitrobenzene at 10℃; for 1h; Stage #2: Benzo[b]thiophene In nitrobenzene for 1h; Friedel-Crafts reaction; Title compound not separated from byproducts; |

-
-
17890-55-0
ethyl benzo[b]thiophene-2-carboxylate

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 90 percent / t-BuOK / 2 h / Ambient temperature 2: 93 percent / 10percent aq. NH4Cl, Zn / ethanol / 1 h / 0 °C View Scheme |
-
-
552-89-6
2-nitro-benzaldehyde

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 94 percent / K2CO3 / dimethylformamide / 1.) 0 deg C, 30 min, 2.) RT, 24 h 2: 90 percent / t-BuOK / 2 h / Ambient temperature 3: 93 percent / 10percent aq. NH4Cl, Zn / ethanol / 1 h / 0 °C View Scheme |

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tetrahydrofuran / 0.33 h / 0 - 5 °C 2: 79 percent / Et3N, SO3*Py / CH2Cl2; dimethylsulfoxide View Scheme |
-
-
147-93-3
Thiosalicylic acid

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1.) LiAlH4, 2.) EtOAc, H2O / 1a.) THF, 0-5 deg C, 1b.) RT, 1h, 2.) 0-5 deg C, 30 min 2: tetrahydrofuran / 0.33 h / 0 - 5 °C 3: 79 percent / Et3N, SO3*Py / CH2Cl2; dimethylsulfoxide View Scheme |
-
-
109-72-8, 29786-93-4
n-butyllithium

-
-
78191-00-1
N-Methoxy-N-methylacetamide

-
-
95-15-8
Benzo[b]thiophene

-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
685-91-6
diethylacetamide

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| In toluene |

| Conditions | Yield |
|---|---|
| In dichloromethane; toluene |

-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
97511-06-3
1-(benzo[b]thiophen-2-yl)-2-bromoethanone

| Conditions | Yield |
|---|---|
| With tetra-N-butylammonium tribromide In methanol; dichloromethane at 20℃; for 15h; Product distribution / selectivity; | 100% |
| With tetrabuthylammonium tribromide In methanol; dichloromethane at 22℃; for 6h; | 95% |
| With copper(ll) bromide In methanol; chloroform at 60℃; for 15h; | 89% |
-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
560108-31-8
(R)-(+)-1-(benzo[b]thiophen-2-yl)ethanol

| Conditions | Yield |
|---|---|
| With borane-THF; (S)-1-methyl-3,3-diphenyl-hexahydropyrrolo[1,2-c][1,3,2]oxazaborole In tetrahydrofuran at 20℃; for 0.666667h; | 100% |
| With dimethylsulfide borane complex; (S)-1-methyl-3,3-diphenyl-hexahydropyrrolo[1,2-c][1,3,2]oxazaborole In tetrahydrofuran; toluene at 20℃; for 29.5h; Inert atmosphere; optical yield given as %ee; | 97% |
| With C38H38IrN4S2(1+)*F6P(1-); ammonium formate; 3-methyl-5-p-methoxyphenyl-1-hydropyrazole In tetrahydrofuran; water at 40℃; for 9h; enantioselective reaction; | 93% |
-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
93-02-7
2,5-dimethoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With pyrrolidine; acetic acid In tetrahydrofuran for 13h; Aldol Condensation; Reflux; Inert atmosphere; | 100% |
-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
51868-95-2
1-(benzo[b]thiophen-2-yl)ethanol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol; water for 1.5h; Heating; | 99% |
| With methanol; sodium tetrahydroborate In water at 5 - 10℃; for 2h; | 90% |
| With sodium tetrahydroborate In methanol at 0℃; for 0.333333h; Reduction; | 87% |
-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| In toluene at 20℃; | 98% |
-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
762-04-9
phosphonic acid diethyl ester

-
-
1370037-12-9
diethyl 1-hydroxy-1-(benzothiophen-2-yl)ethylphosphonate

| Conditions | Yield |
|---|---|
| With {(μ-η5:η1):η1-2-[(2,6-Me2C6H3)NCH2](C4H3N)YN(SiMe3)2}2 In tetrahydrofuran at 20℃; for 0.333333h; Inert atmosphere; | 97% |
-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
64845-11-0
1-(1,1-Dioxo-1H-1λ6-benzo[b]thiophen-2-yl)-ethanone

| Conditions | Yield |
|---|---|
| With Oxone In neat (no solvent) for 1.5h; Milling; Green chemistry; chemoselective reaction; | 97% |
-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
597553-10-1
(S)-(+)-1-(benzo[b]thiophen-2-yl)ethanol

| Conditions | Yield |
|---|---|
| With formic acid; C28H36N3O2RuS; triethylamine at 60℃; for 3h; Inert atmosphere; enantioselective reaction; | 96% |
| With baker's yeast; water In methanol at 20℃; | 92% |
-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
141-78-6
ethyl acetate

-
-
1059697-84-5
(1R)-1-acetoxy-1-(benzo[b]thiophen-2-yl)ethane

| Conditions | Yield |
|---|---|
| With novozyme 435; dicarbonylhydro[2,5-di(trimethylsilyl)-3,4-butylene-1-hydroxy(η5-cyclopentadienyl)]iron; hydrogen In toluene at 90℃; under 750.075 Torr; Schlenk technique; Enzymatic reaction; enantioselective reaction; | 96% |
-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
147396-07-4
2-acetylbenzothiophene oxime

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium acetate In ethanol; water for 4h; Heating; | 95% |
| With hydroxylamine hydrochloride; sodium acetate In ethanol; water Reflux; | 94.2% |
| With hydroxylamine |
-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
183164-38-7
1-((methylthio)methyl)-1H-benzo[d][1,2,3]triazole

| Conditions | Yield |
|---|---|
| Stage #1: 1-((methylthio)methyl)-1H-benzo[d][1,2,3]triazole With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 1h; Stage #2: 2-acetyl benzo[b]thiophene In tetrahydrofuran; hexane at -78℃; for 1h; | 94% |
-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
72824-04-5
2-Allyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

| Conditions | Yield |
|---|---|
| Stage #1: 2-Allyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane With (S)-2-((2-hydroxy-3-(triphenylsilyl)benzyl)amino)-3-methyl-1-(pyrrolidin-1-yl)butan-1-one; sodium t-butanolate In tetrahydrofuran; methanol; toluene at -30℃; for 0.5h; Inert atmosphere; Stage #2: 2-acetyl benzo[b]thiophene In tetrahydrofuran; methanol; toluene at -30℃; for 8h; Inert atmosphere; enantioselective reaction; | 94% |

| Conditions | Yield |
|---|---|
| With ruthenium trichloride; sodium carbonate; Selectfluor at 120℃; for 6h; Mannich Aminomethylation; | 93% |

-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
626-62-0
1-iodocyclohexane

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); 1,3-bis-(diphenylphosphino)propane; caesium carbonate at 110℃; for 36h; Inert atmosphere; Schlenk technique; regioselective reaction; | 92% |
-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
18955-75-4
ethyl 2-diazo-3-oxo-4,4,4-trifluorobutyrate

| Conditions | Yield |
|---|---|
| With copper hydroxide; 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran at 25℃; for 4h; Glovebox; Inert atmosphere; Sealed tube; regioselective reaction; | 92% |
-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
67-68-5
dimethyl sulfoxide

| Conditions | Yield |
|---|---|
| With iodine; sodium nitrite at 90℃; for 12h; | 91% |
-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
102820-40-6
2-(1-ethenyl-2-propenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

| Conditions | Yield |
|---|---|
| Stage #1: 2-(1-ethenyl-2-propenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane With copper (I) acetate; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; sodium t-butanolate In tetrahydrofuran for 0.166667h; Inert atmosphere; Glovebox; Stage #2: 2-acetyl benzo[b]thiophene In tetrahydrofuran at 20℃; Inert atmosphere; Glovebox; Stage #3: With dihydrogen peroxide; sodium hydroxide In tetrahydrofuran at 0℃; for 3h; Inert atmosphere; stereoselective reaction; | A 90% B n/a |

-
-
109-04-6
2-bromo-pyridine

-
-
22720-75-8
2-acetyl benzo[b]thiophene

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromo-pyridine With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 0.5h; Inert atmosphere; Stage #2: 2-acetyl benzo[b]thiophene In tetrahydrofuran at -78 - 25℃; for 18h; Inert atmosphere; | 89% |

| Conditions | Yield |
|---|---|
| Stage #1: hydroxylamine; methyl carbamate With sodium hydroxide In water at 5 - 30℃; Stage #2: 2-acetyl benzo[b]thiophene With methanol; sodium tetrahydroborate In tetrahydrofuran; water at 10 - 40℃; Stage #3: With hydrogenchloride; acetic acid at 40 - 45℃; for 3h; | 88% |

-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
69739-34-0
t-butyldimethylsiyl triflate

| Conditions | Yield |
|---|---|
| Stage #1: 2-acetyl benzo[b]thiophene With triethylamine In dichloromethane at 20 - 25℃; for 1h; Inert atmosphere; Stage #2: t-butyldimethylsiyl triflate In dichloromethane at 20 - 25℃; Inert atmosphere; | 88% |
-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
1826-67-1
vinyl magnesium bromide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0℃; for 0.0833333h; Inert atmosphere; Sonication; | 85% |
-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
64-17-5
ethanol

| Conditions | Yield |
|---|---|
| With diphenyl diselenide; oxygen; toluene-4-sulfonic acid; copper(I) bromide at 130℃; for 9h; Sealed tube; | 85% |
-
-
22720-75-8
2-acetyl benzo[b]thiophene

-
-
108-88-3
toluene

| Conditions | Yield |
|---|---|
| Stage #1: toluene With thianthrene-5-oxide; trifluoromethylsulfonic anhydride In acetonitrile at -20 - 20℃; for 0.666667h; Inert atmosphere; Schlenk technique; Stage #2: 2-acetyl benzo[b]thiophene With palladium diacetate; cesium fluoride; XPhos In N,N-dimethyl-formamide; acetonitrile at 80℃; for 11h; Inert atmosphere; Schlenk technique; | 85% |
2-Acetylbenzo[b]thiophene Specification
The IUPAC name of Ethanone,1-benzo[b]thien-2-yl- is 1-(1-benzothiophen-2-yl)ethanone. With the CAS registry number 22720-75-8, it is also named as 2-Acetylbenzo[b]thiophene. The product's categories are Fluorobenzene; Thiophene & Benzothiophene. Besides, it is white to light yellow crystal powder, which should be stored in sealed container in cool and dry place. In addition, its molecular formula is C10H8OS and molecular weight is 176.23.
The other characteristics of this product can be summarized as: (1)EINECS: 245-177-7; (2)ACD/LogP: 3.83; (3)# of Rule of 5 Violations: 0; (4)ACD/LogD (pH 5.5): 3.83; (5)ACD/LogD (pH 7.4): 3.83; (6)ACD/BCF (pH 5.5): 476.71; (7)ACD/BCF (pH 7.4): 476.71; (8)ACD/KOC (pH 5.5): 2875.41; (9)ACD/KOC (pH 7.4): 2875.41; (10)#H bond acceptors: 1; (11)#H bond donors: 0; (12)#Freely Rotating Bonds: 1; (13)Polar Surface Area: 45.31 Å2; (14)Index of Refraction: 1.646; (15)Molar Refractivity: 52.5 cm3; (16)Molar Volume: 144.5 cm3; (17)Polarizability: 20.81×10-24cm3; (18)Surface Tension: 47.4 dyne/cm; (19)Density: 1.219 g/cm3; (20)Flash Point: 138 °C; (21)Melting Point: 86-88 °C; (22)Enthalpy of Vaporization: 54.49 kJ/mol; (23)Boiling Point: 304.5 °C at 760 mmHg; (24)Vapour Pressure: 0.00087 mmHg at 25 °C.
Preparation of Ethanone,1-benzo[b]thien-2-yl-: this chemical can be prepared by 1-(2-Hydroxymethyl-phenylsulfanyl)-propan-2-one.
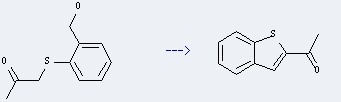
This reaction needs Et3N, SO3*Py, CH2Cl2 and Dimethylsulfoxide. The yield is 79 %.
Uses of Ethanone,1-benzo[b]thien-2-yl-: this chemical is a novel anti-osteoporosis agent. It is also used as drug intermediates for the synthesis of zileuton. Furthermore, it can be used to produce 1-Benzo[b]thiophen-2-yl-2-bromo-ethanone.

This reaction needs (n-Bu)4NBr3, CH2Cl2 and Methanol at temperature of 22 °C for 6 hours. The yield is 95 %.
When you are using this chemical, please be cautious about it as the following: it is harmful by inhalation, in contact with skin and if swallowed. You should wear suitable protective clothing when use it.
People can use the following data to convert to the molecule structure.
(1)SMILES: O=C(c2sc1ccccc1c2)C
(2)InChI: InChI=1/C10H8OS/c1-7(11)10-6-8-4-2-3-5-9(8)12-10/h2-6H,1H3
(3)InChIKey: SGSGCQGCVKWRNM-UHFFFAOYAK
(4)Std. InChI: InChI=1S/C10H8OS/c1-7(11)10-6-8-4-2-3-5-9(8)12-10/h2-6H,1H3
(5)Std. InChIKey: SGSGCQGCVKWRNM-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View