-
Name
2-Cyanophenol
- EINECS 210-259-3
- CAS No. 611-20-1
- Article Data247
- CAS DataBase
- Density 1.22 g/cm3
- Solubility soluble in water
- Melting Point 92-95 °C(lit.)
- Formula C7H5NO
- Boiling Point 273.1 °C at 760 mmHg
- Molecular Weight 119.123
- Flash Point 109.6 °C
- Transport Information UN 3276
- Appearance white to light yellow crystal powder
- Safety 26-36-45
- Risk Codes 22-36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms Salicylonitrile(6CI,7CI,8CI);2-Hydroxybenzonitrile;NSC 53558;Salicylnitrile;o-Cyanophenol;o-Hydroxybenzonitrile;
- PSA 44.02000
- LogP 1.26388
Synthetic route
-
-
21013-96-7
(E)-2-hydroxylbenzaldehyde oxime

-
-
611-20-1
salicylonitrile

| Conditions | Yield |
|---|---|
| With 1,3,5-trichloro-2,4,6-triazine In N,N-dimethyl-formamide at 20℃; for 4h; Beckmann rearrangement; | 100% |
| With di-isopropyl azodicarboxylate; triphenylphosphine In dichloromethane at 20℃; for 1h; Solvent; Mitsunobu Displacement; Inert atmosphere; | 97% |
| With aluminum oxide; methanesulfonyl chloride at 100℃; for 0.0833333h; | 94% |

| Conditions | Yield |
|---|---|
| With 2-(methylsulfonyl)ethyl alcohol; sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; | 100% |
| With methyl propargyl alcohol; potassium tert-butylate In dimethyl sulfoxide at 125℃; for 0.0333333h; microwave irradiation; | 73% |
| Multi-step reaction with 3 steps 1.1: 94 percent / NaH / dimethylformamide / 3 h / 70 °C 2.1: 7.08 g / ICl; K2CO3 / CH2Cl2 / 1 h / Heating 3.1: iPrMgCl / tetrahydrofuran / 0.52 h / -78 °C 3.2: ZnCl2 / tetrahydrofuran / -78 - 20 °C 3.3: Pd(PPh3)4 / tetrahydrofuran; dimethylformamide / 18 h / 20 °C View Scheme |
-
-
218797-78-5
2-(tert-butoxy)benzonitrile

-
-
611-20-1
salicylonitrile

| Conditions | Yield |
|---|---|
| With sodium iodide; cerium(III) chloride In acetonitrile at 40℃; for 6h; | 100% |
| Multi-step reaction with 2 steps 1: potassium tert-butylate / 1,4-dioxane / 16 h / 80 °C / Inert atmosphere; Sealed tube 2: potassium tert-butylate; 18-crown-6 ether; water / 1,4-dioxane / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: potassium tert-butylate / 1,4-dioxane / 16 h / 80 °C / Inert atmosphere; Sealed tube 2: potassium tert-butylate / 1,4-dioxane / 16 h / 80 °C / Inert atmosphere; Sealed tube; Glovebox 3: potassium tert-butylate; 18-crown-6 ether; water / 1,4-dioxane / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: potassium tert-butylate / 1,4-dioxane / 16 h / 80 °C / Inert atmosphere; Sealed tube 2: potassium tert-butylate; 18-crown-6 ether / 1,4-dioxane / 16 h / 20 °C / Inert atmosphere; Sealed tube 3: potassium tert-butylate; 18-crown-6 ether; water / 1,4-dioxane / 16 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| With chlorosulfonic acid In toluene at 90℃; for 0.5h; | 99% |
| With oxalyl dichloride; Tropone; 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 50℃; for 0.166667h; Schlenk technique; Inert atmosphere; | 99% |
| With acetyl chloride; zinc(II) oxide for 0.5h; Heating; | 95% |
-
-
97960-34-4
2-(prop-2'-enyloxy)benzonitrile

-
-
611-20-1
salicylonitrile

| Conditions | Yield |
|---|---|
| With tetrabutylammonium tetrafluoroborate; tris(2,2'-bipyridine)nickel(II) tetrafluoroborate In N,N-dimethyl-formamide Ambient temperature; electrolysis: Mg rod anode, carbon fibre cathode, const. current 60 mA; | 99% |
-
-
65211-56-5
2-(prop-2-yn-1-yloxy)benzonitrile

-
-
611-20-1
salicylonitrile

| Conditions | Yield |
|---|---|
| tris(2,2'-bipyridine)nickel(II) tetrafluoroborate In N,N-dimethyl-formamide Ambient temperature; electrolysis; | 99% |

| Conditions | Yield |
|---|---|
| With phosgene; L-lysine In 1,2-dichloro-ethane at 80℃; for 4h; Reagent/catalyst; Temperature; | 98.8% |
| With phosgene; cyanex-921 In 1,2-dichloro-ethane for 4h; Reagent/catalyst; Solvent; Reflux; | 98.9% |
| With thionyl chloride In 5,5-dimethyl-1,3-cyclohexadiene at 105℃; for 7h; Concentration; Temperature; Solvent; Reagent/catalyst; | 97.8% |

| Conditions | Yield |
|---|---|
| With ammonia; boron phosphate at 400℃; for 0.000277778h; | 98% |
| Multi-step reaction with 2 steps 1: alcohol; ammonia / 100 °C 2: phosphorus pentoxide View Scheme | |
| Multi-step reaction with 2 steps 1: alcohol; ammonia / 100 °C 2: phosphorus pentoxide View Scheme | |
| Multi-step reaction with 3 steps 1: alcohol; ammonia / 100 °C 2: P2S5 3: bei der Destillation unter vermindertem Druck View Scheme |

-
-
611-20-1
salicylonitrile

| Conditions | Yield |
|---|---|
| With zirconium(IV) chloride In nitromethane for 0.0333333h; Heating; | 97% |
-
-
114536-52-6
salicylonitril; ammonium salt

-
-
611-20-1
salicylonitrile

| Conditions | Yield |
|---|---|
| With ammonia; N,N-dimethyl-formamide; Xylene; mixture of at 420℃; Product distribution / selectivity; | 97% |
| With sulfuric acid; ammonia; water at 60℃; pH=3 - 4; Product distribution / selectivity; | 96% |
| With ammonia; Xylene; mixture of at 420℃; Product distribution / selectivity; | 94% |
| Stage #1: salicylonitril; ammonium salt With ammonia; water at 15 - 60℃; Stage #2: In N,N-dimethyl-formamide at 60℃; under 37.5038 - 75.0075 Torr; Product distribution / selectivity; | 72% |

| Conditions | Yield |
|---|---|
| With tris(6,6'-diamino-2,2'-bipyridine); 4,4-diphenyl-1,3,5,7,8-pentamethyl-2,6-diethyl-4-bora-3a,4a-diaza-s-indacene; Br2Ni*3H2O; water; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide; acetonitrile at 20℃; for 24h; Glovebox; Irradiation; Inert atmosphere; | 97% |
| With potassium hydroxide; water; polyaniline-supported palladium In 1,4-dioxane at 100℃; for 8h; | 80% |
| With oxygen; triethylamine; sodium iodide In acetonitrile at 32℃; for 24h; Schlenk technique; UV-irradiation; | 64% |
| With formic acid; oxygen; triethylamine; copper(ll) bromide In acetonitrile at 20℃; for 48h; Irradiation; | 61% |

| Conditions | Yield |
|---|---|
| With iron(III) chloride; hydroxylamine hydrochloride In N,N-dimethyl-formamide for 4h; Reflux; | 96% |
| With hydroxylamine hydrochloride In acetonitrile at 70℃; for 15h; | 96% |
| With trifluorormethanesulfonic acid; trimethylsilylazide In acetonitrile at 25℃; for 0.00277778h; Schmidt Reaction; Flow reactor; | 96% |
-
-
64-17-5
ethanol

-
-
60451-91-4
benzo[d]isoxazol-3-yl(phenyl)methanone

-
A
-
611-20-1
salicylonitrile

-
B
-
93-89-0
benzoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium hydroxide for 16h; Ambient temperature; | A 95% B 90% |

-
-
13589-72-5
5-chloro-2-hydroxybenzonitrile

-
-
611-20-1
salicylonitrile

| Conditions | Yield |
|---|---|
| With isopropyl alcohol Substitution; Photolysis; | 95% |

| Conditions | Yield |
|---|---|
| With Aloe vera mediated silver nanoparticles In water at 50℃; for 1h; Green chemistry; | 94% |
-
-
51-17-2
benzoimidazole

-
-
172732-52-4
o-cyanophenylboronic acid-1,3-propylene glycol ester

-
A
-
611-20-1
salicylonitrile

-
B
-
100-47-0
benzonitrile

-
C
-
25699-93-8
2-(1H-benzo[d]imidazol-1-yl)benzonitrile

| Conditions | Yield |
|---|---|
| With pyridine; water; copper diacetate In N,N-dimethyl-formamide at 30℃; for 24h; Kinetics; Product distribution; Further Variations:; Reaction partners; amount of water; | A n/a B n/a C 93% |

-
-
138642-62-3
2-Cyanophenylboronic acid

-
-
611-20-1
salicylonitrile

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In 1,4-dioxane at 20℃; under 760.051 Torr; for 28h; Irradiation; Green chemistry; | 93% |
| With 1,10-Phenanthroline; copper(II) sulfate; potassium hydroxide In water at 20℃; for 5h; | 82% |
| With C38H34Cl2NP3Pd; oxygen; triethylamine In chloroform at 24.84℃; for 24h; | 76% |

| Conditions | Yield |
|---|---|
| With sodium methylate; sodium ethanolate In methanol; ethanol at 170℃; under 760.051 Torr; | 93% |
| With oxygen; triethylamine; sodium iodide In acetonitrile at 32℃; for 24h; Schlenk technique; UV-irradiation; | 52% |

| Conditions | Yield |
|---|---|
| In toluene | 92.5% |

| Conditions | Yield |
|---|---|
| With 1,3-dimethyl-2-imidazolidinone; lithium diisopropyl amide In tetrahydrofuran; n-heptane; ethylbenzene at 185℃; for 12h; further reagent: NaN(SiMe3)2; | 91% |
| With aluminium(III) iodide; N,N-dimethyl-formamide dimethyl acetal In acetonitrile at 80℃; for 18h; | 10% |
| With aluminium(III) iodide; N,N-dimethyl-formamide dimethyl acetal In acetonitrile at 80℃; for 18h; | 10% |

-
-
611-20-1
salicylonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 2-(2-trimethylsilanylethoxy)benzonitrile With cesium fluoride In N,N-dimethyl-formamide at 60℃; for 1h; Inert atmosphere; Stage #2: With water In N,N-dimethyl-formamide Inert atmosphere; | 91% |

| Conditions | Yield |
|---|---|
| With ammonia; boron phosphate at 400℃; for 0.000277778h; | 90% |
| With sodium hexamethyldisilazane In tetrahydrofuran at 185℃; for 24h; | 85% |
| Multi-step reaction with 2 steps 1: alcohol; ammonia / 100 °C 2: phosphorus pentoxide View Scheme |

| Conditions | Yield |
|---|---|
| With aluminum oxide; hydroxylamine hydrochloride; methanesulfonyl chloride at 100℃; for 0.583333h; | 90% |
| With Mont KSF; hydroxylamine hydrochloride; silica gel for 0.0666667h; microwave irradiation; | 89% |
| With 1,3-dimethyl-2-imidazolidinone; sodium hexamethyldisilazane In tetrahydrofuran at 185℃; for 12h; | 88% |

| Conditions | Yield |
|---|---|
| With racemic 2-di-tert-butylphosphino-1,1'-binaphthyl; palladium(II) trifluoroacetate; zinc In N,N-dimethyl acetamide at 95℃; for 3h; | 88.4% |
| With sulfuric acid; palladium diacetate; zinc; XPhos In 2,4-dichlorophenoxyacetic acid dimethylamine at 120℃; for 2h; Inert atmosphere; | 98 %Chromat. |

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; ammonia; magnesium sulfate In tetrahydrofuran; isopropyl alcohol at 20℃; for 18h; | 87% |

| Conditions | Yield |
|---|---|
| With potassium phosphate In N,N-dimethyl-formamide at 120℃; for 2h; | 87% |

| Conditions | Yield |
|---|---|
| tungsten(VI) oxide at 145 - 400℃; under 0.025 Torr; for 1.23333h; Pyrolysis; | A 85% B 8% |
| With H3PO4 immobilized on aluminium oxide at 480℃; | A 50.78 %Chromat. B 24.12 %Chromat. |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydroxide; boron trichloride; aluminium chloride In 1,2-dichloro-ethane | 84% |
| With hydrogenchloride; sodium hydroxide; boron tribromide; aluminium chloride In 1,2-dichloro-ethane | 79% |
| With boron trichloride; sodium carbonate Product distribution; various phenols; | |
| With sodium hydroxide; aluminium trichloride; boron trichloride 1.) dichloroethane, 80 deg C, 3 h, 2.) 80 deg C, 30 min; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With sodium hexamethyldisilazane In tetrahydrofuran at 185℃; for 24h; | 83% |

-
-
611-20-1
salicylonitrile

| Conditions | Yield |
|---|---|
| at 40℃; under 0.5 Torr; for 3h; | 82% |
-
-
611-20-1
salicylonitrile

-
-
70-11-1
α-bromoacetophenone

-
-
49615-93-2
(3-amino-benzofuran-2-yl)-phenyl-methanone

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 60℃; for 18h; Inert atmosphere; | 100% |
| With potassium carbonate In acetone at 60℃; for 18h; | 100% |
| With sodium carbonate In N,N-dimethyl-formamide at 80℃; for 1h; Inert atmosphere; | 96% |
-
-
611-20-1
salicylonitrile

-
-
96-32-2
bromoacetic acid methyl ester

-
-
34844-79-6
methyl 2-(2-cyanophenoxy)ethanoate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 25℃; for 24h; Alkylation; | 100% |
| With potassium carbonate In acetone for 7h; Reflux; | 94% |
| With potassium carbonate In acetone at 20℃; | 61.5% |
-
-
611-20-1
salicylonitrile

-
-
120925-50-0
(R)-2-methyloctan-1-ol

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate | 100% |
-
-
611-20-1
salicylonitrile

-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
609804-44-6
tert-butyl 2-(2-cyanophenoxy)acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 60℃; for 16h; | 100% |
| With potassium carbonate In acetone for 2h; Heating / reflux; | |
| With potassium carbonate In acetone for 2h; Heating / reflux; |
-
-
611-20-1
salicylonitrile

-
-
109384-19-2
t-butyl 4-hydroxy piperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: salicylonitrile; t-butyl 4-hydroxy piperidine-1-carboxylate With di-tert-butyl-diazodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 18h; Stage #2: With hydrogenchloride; water In 1,4-dioxane at 20℃; for 18h; | 100% |
-
-
131860-97-4
(E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate

-
-
611-20-1
salicylonitrile

-
-
215934-32-0, 131860-33-8
azoxystrobin

| Conditions | Yield |
|---|---|
| With potassium carbonate; quinuclidine hydrochloride In 4-methyl-2-pentanone at 60 - 80℃; for 0.5h; Product distribution / selectivity; | 100% |
| With potassium carbonate; 1,4-diaza-bicyclo[2.2.2]octane In N,N-dimethyl-formamide at 48 - 65℃; for 1h; Product distribution / selectivity; | 98.3% |
| Stage #1: (E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate; salicylonitrile In N,N-dimethyl-formamide at 60℃; for 0.0833333h; Stage #2: 1,4-diaza-bicyclo[2.2.2]octane In N,N-dimethyl-formamide at 60℃; for 0.0833333h; Stage #3: With potassium carbonate In N,N-dimethyl-formamide at 60 - 89℃; for 0.166667h; Product distribution / selectivity; | 97.1% |
-
-
611-20-1
salicylonitrile

-
-
584-08-7
potassium carbonate

-
-
96-32-2
bromoacetic acid methyl ester

-
B
-
34844-79-6
methyl 2-(2-cyanophenoxy)ethanoate

| Conditions | Yield |
|---|---|
| In acetone | A 100% B n/a |


| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran | 100% |
| With potassium carbonate; N,N-dimethyl-formamide In toluene at 50℃; for 16h; Inert atmosphere; | 94% |
| With potassium carbonate In N,N-dimethyl-formamide | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: salicylonitrile With sodium hydride In 1,2-dimethoxyethane; mineral oil at 0℃; for 0.166667h; Stage #2: dimethylamino sulfonyl chloride In 1,2-dimethoxyethane; mineral oil at 0 - 20℃; for 11h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Reflux; | 100% |
-
-
611-20-1
salicylonitrile

-
-
622-58-2
p-Tolylisocyanate

-
-
88220-35-3
2-<(4-methylphenyl)aminocarbonyloxy>benzonitrile

| Conditions | Yield |
|---|---|
| With triethylamine In diethyl ether | 99% |
-
-
611-20-1
salicylonitrile

-
-
52-89-1
l-cysteine hydrochloride

-
-
115921-06-7
(R)-2-(2-hydroxyphenyl)-4,5-dihydro-1,3-thiazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In methanol; aq. phosphate buffer for 72h; pH=6; Reflux; | 99% |
| Stage #1: salicylonitrile; l-cysteine hydrochloride With sodium hydrogencarbonate In ethanol for 0.5h; Heating; Stage #2: With piperidine In ethanol for 12h; pH=9; Heating; Stage #3: With hydrogenchloride In ethanol pH=1.5; | 67% |
-
-
611-20-1
salicylonitrile

-
-
5445-17-0
Methyl 2-bromopropionate

-
-
182964-51-8
2-[1-(methoxycarbonyl)ethoxy]benzonitrile

| Conditions | Yield |
|---|---|
| In water; N,N-dimethyl-formamide | 99% |
-
-
611-20-1
salicylonitrile

-
-
173206-13-8
4,5-dichloro-2-(tetrahydro-2H-pyran-2-yl)-3(2H)-pyridazinone

-
-
1191454-37-1
2-[5-chloro-6-oxo-1-(tetrahydro-pyran-2-yl)-1,6-dihydro-pyridazin-4-yloxy]-benzonitrile

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 2h; Reflux; | 99% |
-
-
611-20-1
salicylonitrile

-
-
693-25-4
n-pentylmagnesium bromide

-
-
3226-15-1
1-(2-hydroxyphenyl)hexan-1-one

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 70℃; Inert atmosphere; | 99% |
| Stage #1: salicylonitrile; n-pentylmagnesium bromide In tetrahydrofuran Inert atmosphere; Heating; Stage #2: With hydrogenchloride; water In tetrahydrofuran Cooling with ice; |

| Conditions | Yield |
|---|---|
| With potassium carbonate at 65℃; for 18h; Sealed tube; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 56℃; | 98.5% |
| Stage #1: salicylonitrile With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 1h; Stage #2: Chloroacetamide In N,N-dimethyl-formamide at 80℃; for 3h; | |
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; |
-
-
611-20-1
salicylonitrile

-
-
51449-77-5
2-(1H-tetrazol-5-yl)phenol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium azide; water; triethylamine hydrochloride In toluene at 95 - 99℃; for 6h; | 98% |
| 98% | |
| With sodium azide at 120℃; for 0.333333h; | 98% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 50℃; for 3h; | 98% |
| With potassium carbonate In acetone for 2h; Heating; | 88.6% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 12h; | 87% |

| Conditions | Yield |
|---|---|
| With potassium carbonate for 6h; Heating; | 98% |
-
-
611-20-1
salicylonitrile

-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
138313-23-2
2-<(trifluoromethanesulfonyl)oxy>benzonitrile

| Conditions | Yield |
|---|---|
| With pyridine 1.) 0 deg C, 5 min, 2.) r.t., 25 h; | 98% |
| With pyridine In dichloromethane at 0 - 25℃; Inert atmosphere; | 97% |
| With pyridine In dichloromethane at 20℃; for 0.5h; | 95% |
-
-
611-20-1
salicylonitrile

-
-
215934-32-0, 131860-33-8
azoxystrobin

| Conditions | Yield |
|---|---|
| Stage #1: (E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 0.0833333h; Stage #2: salicylonitrile In N,N-dimethyl-formamide at 60℃; for 5h; Stage #3: 1,4-diaza-bicyclo[2.2.2]octane In N,N-dimethyl-formamide at 60 - 89℃; for 0.166667h; Product distribution / selectivity; | 98% |
| Stage #1: (E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate With potassium carbonate In Isopropyl acetate at 40℃; for 0.166667h; Stage #2: salicylonitrile In Isopropyl acetate for 0.166667h; Stage #3: 1,4-diaza-bicyclo[2.2.2]octane In Isopropyl acetate at 40 - 45℃; for 0.5h; Product distribution / selectivity; | 97.5% |
| Stage #1: (E)-methyl 2-[2-(6-chloropyrimidin-4-yloxy)phenyl]-3-methoxy-propenoate With potassium carbonate In Isopropyl acetate at 40℃; for 0.166667h; Stage #2: 1,4-diaza-bicyclo[2.2.2]octane In Isopropyl acetate for 0.166667h; Stage #3: salicylonitrile In Isopropyl acetate at 40 - 45℃; for 0.333333 - 0.5h; Product distribution / selectivity; | 94.5% |
2-Cyanophenol Specification
The 2-Cyanophenol, with the CAS registry number 611-20-1, is also known as Salicylonitrile. It belongs to the product categories of Aromatic Nitriles; Miscellaneous. Its EINECS registry number is 210-259-3. This chemical's molecular formula is C7H5NO and molecular weight is 119.12. What's more, its IUPAC name is called 2-Hydroxybenzonitrile. It should be stored in a cool, dry and well-ventilated place.
Physical properties about 2-Cyanophenol are: (1)ACD/LogP: 1.026; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.02; (4)ACD/LogD (pH 7.4): 0.60; (5)ACD/BCF (pH 5.5): 3.47; (6)ACD/BCF (pH 7.4): 1.32; (7)ACD/KOC (pH 5.5): 84.32; (8)ACD/KOC (pH 7.4): 31.98; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 44.02 Å2; (13)Index of Refraction: 1.591; (14)Molar Refractivity: 32.849 cm3; (15)Molar Volume: 97.264 cm3; (16)Surface Tension: 57.85 dyne/cm; (17)Density: 1.225 g/cm3; (18)Flash Point: 109.584 °C; (19)Enthalpy of Vaporization: 53.202 kJ/mol; (20)Boiling Point: 273.109 °C at 760 mmHg; (21)Vapour Pressure: 0.0030 mmHg at 25 °C.
Preparation of 2-Cyanophenol: this chemical can be prepared by Salicylaldehyde-(E)-oxime. This reaction needs reagent 2,4,6-trichloro[1,3,5]triazine and solvent dimethylformamide at temperature of 20 °C. The reaction time is 4 hours. The yield is 100 %.
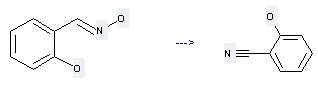
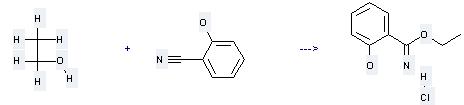
Uses of 2-Cyanophenol: (1) it is used as bunitrolol intermediates; (2) it is used to produce other chemicals. For example, it can react with ethanol to get salicylimidic acid ethyl ester; hydrochloride. The reaction occurs with reagent HCl and solvent benzene at temperature of 20 °C. The reaction time is 15 days. The yield is 37 %.
When you are dealing with this chemical, you should be very careful. This chemical is inflammation to the skin, eyes and respiratory system or other mucous membranes. It is harmful by inhalation, in contact with skin and if swallowed. Therefore, you should wear suitable protective clothing. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice. And in case of accident or if you feel unwell seek medical advice immediately.
You can still convert the following datas into molecular structure:
(1) SMILES: N#Cc1ccccc1O
(2) InChI: InChI=1S/C7H5NO/c8-5-6-3-1-2-4-7(6)9/h1-4,9H
(3) InChIKey: CHZCERSEMVWNHL-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View