-
Name
2-Methylaminoethanol
- EINECS 203-710-0
- CAS No. 109-83-1
- Article Data75
- CAS DataBase
- Density 0.89 g/cm3
- Solubility miscible with water
- Melting Point -3 °C
- Formula C3H9NO
- Boiling Point 155.5 °C at 760 mmHg
- Molecular Weight 75.1106
- Flash Point 72.8 °C
- Transport Information UN 2735 8/PG 2
- Appearance viscous colourless or light yellow liquid
- Safety 26-36/37/39-45
- Risk Codes 21/22-34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms (2-Hydroxyethyl)methylamine;(Hydroxyethyl)methylamine;2-Hydroxy-N-methylethylamine;2-Hydroxyethyl-N-methylamine;2-Methylamino-1-ethanol;2-N-Monomethylaminoethanol;Amietol M 11;AminoAlcohol MMA;Methyl(2-hydroxyethyl)amine;Methyl(hydroxyethyl)amine;Methyl(b-hydroxyethyl)amine;Methylaminoethanol;Methylethanolamine;Methylethylolamine;Monomethylaminoethanol;Monomethylethanolamine;N-Methyl-N-(2-hydroxyethyl)amine;N-Methylaminoethanol;N-Methylmonoethanolamine;N-Monomethylaminoethanol;N-Monomethylethanolamine;NMEA;NSC 62776;Yubao;b-(Methylamino)ethanol;
- PSA 32.26000
- LogP -0.41100
Synthetic route
-
-
75-21-8
oxirane

-
-
74-89-5
methylamine

-
A
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
B
-
105-59-9
N-Methyldiethanolamine

-
C
-
68998-54-9
2,2'-((1-hydroxypropan-2-yl)azanediyl)bis(ethan-1-ol)

| Conditions | Yield |
|---|---|
| water at 62.84 - 71.84℃; under 7500.75 Torr; for 0.2h; Heating / reflux; | A 16.7% B 83.3% C n/a |
| at 66.84 - 76.84℃; under 9000.9 Torr; for 0.116667h; Heating / reflux; | A 63.5% B 36.4% C 0.02% |
| water at 61.54 - 63.34℃; under 7500.75 Torr; for 0.2h; Heating / reflux; | |
| at 66.34℃; under 9000.9 Torr; for 0.116667h; Heating / reflux; |


| Conditions | Yield |
|---|---|
| With ammonium formate; palladium on activated charcoal In methanol for 0.166667h; Heating; | 83% |
| With hydrogen; Pd/C (5percent) In ethanol at 20℃; for 2h; | 67% |
| With hydrazine hydrate; palladium on activated charcoal In ethanol for 2h; Heating; | 42% |
-
-
39931-24-3
4-methyl-1-oxa-4-azaspiro<4.5>decane

-
A
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
B
-
51265-33-9
1-methyl-4,5,6,7-tetrahydro-1H-indole

-
C
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With potassium hydroxide Heating; CH3ONa sa reagent; | A n/a B 72% C n/a |


| Conditions | Yield |
|---|---|
| Stage #1: formaldehyd; ethanolamine With zinc(II) chloride In dichloromethane at 20℃; for 1h; Stage #2: With sodium tetrahydroborate In dichloromethane for 18h; | 72% |
| Stage #1: formaldehyd; ethanolamine Stage #2: With sodium tetrahydroborate |

| Conditions | Yield |
|---|---|
| With methyllithium In tetrahydrofuran for 1h; Product distribution; Ambient temperature; further reagents investigated; | 66% |
-
-
107-21-1
ethylene glycol

-
-
74-89-5
methylamine

-
A
-
106-58-1
N,N'-dimethylpiperazine

-
B
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
C
-
110-70-3
N,N`-dimethylethylenediamine

| Conditions | Yield |
|---|---|
| With 1-methyl-pyrrolidin-2-one; tris(triphenylphosphine)ruthenium(II) chloride at 130℃; Product distribution; Mechanism; other primary amines, other temperatures, other catalyst; | A 33% B 4% C 44% |


| Conditions | Yield |
|---|---|
| In ethanol; water at 30 - 40℃; | 30% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; aluminum nickel for 26.1h; | 28% |
-
-
67-56-1
methanol

-
-
141-43-5
ethanolamine

-
A
-
151-56-4
ethyleneimine

-
B
-
110-85-0
piperazine

-
C
-
280-57-9
1,4-diaza-bicyclo[2.2.2]octane

-
D
-
109-83-1
(2-hydroxyethyl)(methyl)amine

| Conditions | Yield |
|---|---|
| With Cs-P-Si mixed-oxide at 300℃; under 750.06 Torr; Title compound not separated from byproducts; | A 37 % Chromat. B n/a C n/a D 6% |

-
-
67-56-1
methanol

-
-
141-43-5
ethanolamine

-
A
-
110-85-0
piperazine

-
B
-
280-57-9
1,4-diaza-bicyclo[2.2.2]octane

-
C
-
109-83-1
(2-hydroxyethyl)(methyl)amine

| Conditions | Yield |
|---|---|
| With H-β-zeolite at 250℃; under 750.06 Torr; Title compound not separated from byproducts; | A n/a B n/a C 2% |

-
-
94713-16-3
4,5-epoxy-3-methoxy-17-methyl-morphin-6-en-8-one

-
-
108-24-7
acetic anhydride

-
A
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
B
-
7463-47-0
1,5-diacetoxy-4-[2-(acetyl-methyl-amino)-ethyl]-6-methoxy-phenanthrene

-
-
115-37-7, 23979-17-1, 23979-19-3
(+-)-thebaine

-
-
127-09-3
sodium acetate

-
-
108-24-7
acetic anhydride

-
A
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
B
-
47192-97-2
3,6-Dimethoxy-4-acetoxyphenanthrene

-
-
75-21-8
oxirane

-
-
74-89-5
methylamine

-
A
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
B
-
105-59-9
N-Methyldiethanolamine

| Conditions | Yield |
|---|---|
| unter starker Kuehlung; |


| Conditions | Yield |
|---|---|
| With sulfuric acid |
-
-
110-86-1
pyridine

-
-
108-01-0
2-(N,N-dimethylamino)ethanol

-
-
94-36-0
dibenzoyl peroxide

-
A
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
B
-
50-00-0
formaldehyd


| Conditions | Yield |
|---|---|
| With tetrahydrofuran; lithium aluminium tetrahydride |
-
-
13200-60-7
Sarcosine ethyl ester

-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

| Conditions | Yield |
|---|---|
| With ethanol; nickel at 100℃; under 257428 Torr; Hydrogenation; |
-
-
22074-92-6
methyl-carbamic acid-(2-chloro-ethyl ester)

-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

| Conditions | Yield |
|---|---|
| With potassium hydroxide | |
| With sodium hydroxide und nachfolgendes Aufbewahren oder Erwaermen auf 90-100grad; |
-
-
91247-76-6
2-(methyl-piperonyl-amino)-ethanol

-
-
108-24-7
acetic anhydride

-
A
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
B
-
495-76-1
piperonol

| Conditions | Yield |
|---|---|
| nachfolgend Erhitzen mit alkoh. Kalilauge; |


| Conditions | Yield |
|---|---|
| With tetrahydrofuran; lithium aluminium tetrahydride |

| Conditions | Yield |
|---|---|
| With benzoyl chloride at 0℃; | |
| With sodium acetate; acetic anhydride durch Spaltung; |
-
-
50-00-0
formaldehyd

-
-
141-43-5
ethanolamine

-
A
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
B
-
108-01-0
2-(N,N-dimethylamino)ethanol

| Conditions | Yield |
|---|---|
| durch unter azeotropem Abdestillieren des gebildeten Wassers mit Benzol und Hydrierung des Reaktionsprodukts an Raney-Nickel bei 80grad/140 at; |

-
-
75-58-1
tertamethylammonium iodide

-
-
141-43-5
ethanolamine

-
A
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
B
-
75-50-3
trimethylamine

| Conditions | Yield |
|---|---|
| at 154℃; Rate constant; |


| Conditions | Yield |
|---|---|
| With water at 110℃; | |
| In ethanol at 25℃; for 15h; |
-
-
108-24-7
acetic anhydride

-
-
467-13-0, 65494-91-9, 105815-00-7
4,5-epoxy-3-methoxy-17-methyl-morphin-7-en-6-one

-
A
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
B
-
16654-23-2
4,6-diacetoxy-3-methoxy-phenanthrene

-
-
78456-50-5
2-(4-methylphenyl)-2,3-dimethyl-1,3-oxazolidine

-
A
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
B
-
77-78-1
dimethyl sulfate

| Conditions | Yield |
|---|---|
| With sodium hydroxide; acetic acid buffer In acetonitrile at 25℃; Rate constant; Mechanism; var. time, var. buffer, var. pH; |
-
-
141-43-5
ethanolamine

-
-
77-78-1
dimethyl sulfate

-
A
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
B
-
108-01-0
2-(N,N-dimethylamino)ethanol

| Conditions | Yield |
|---|---|
| With calcium hydride 1.) THF, room temp., 1 h; 2.) room temp, 1 h; Yield given. Multistep reaction; | |
| With lithium hydride 1.) THF, room temp., 1 h; 2.) room temp., 1 h; Yield given. Multistep reaction; |


| Conditions | Yield |
|---|---|
| With perchloric acid at 10.1℃; Kinetics; Thermodynamic data; ΔH(excit.); -ΔS(excit.); solvent deuterium isotope effects; different temperatures; |

| Conditions | Yield |
|---|---|
| With perchloric acid at 10.1℃; Kinetics; Thermodynamic data; ΔH(excit.); -ΔS(excit.); different temperatures; |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
40052-63-9
1-bromo-2-(N-methylamino)ethane hydrobromide

| Conditions | Yield |
|---|---|
| With sulfurous dibromide; N,N-dimethyl-formamide In toluene at 50℃; | 100% |
| With hydrogen bromide at 4 - 123℃; | 83% |
| With hydrogen bromide In water at 4℃; | 60.4% |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
292638-85-8
acrylic acid methyl ester

-
-
20517-44-6
N-(2-hydroxy-ethyl)-N-methyl-β-alanine methyl ester

| Conditions | Yield |
|---|---|
| for 3h; Sonication; Schlenk technique; Inert atmosphere; | 100% |
| With methanol |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
66046-61-5
2-chloro-3-methyl-1-oxa-3-aza-2-phosphacyclopentane 2-oxide

| Conditions | Yield |
|---|---|
| With triethylamine; trichlorophosphate In dichloromethane for 17h; Ambient temperature; | 100% |
| With triethylamine; trichlorophosphate In chloroform at 25 - 30℃; for 0.5h; | 86% |
| With triethylamine; trichlorophosphate In dichloromethane | |
| With triethylamine; trichlorophosphate In diethyl ether at 0℃; for 2h; | |
| With triethylamine In dichloromethane |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
57561-39-4
methyl(2-hydroxyethyl)carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; Condensation; | 100% |
| In dichloromethane at 20℃; for 1h; | 100% |
| In tetrahydrofuran at 20℃; for 4h; | 100% |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
106-89-8
epichlorohydrin

-
-
63125-83-7
1-Chlor-2-hydroxy-3-(N-methyl-N-2-hydroxyethyl)-amino-propan

| Conditions | Yield |
|---|---|
| at 40 - 70℃; | 100% |
| In water at 13 - 34℃; for 1.41667h; | |
| In isopropyl alcohol |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
624-28-2
2,5-dibromopyridine

-
-
149806-47-3
2-[(5-bromopyridin-2-yl)methylamino]ethanol

| Conditions | Yield |
|---|---|
| at 150℃; for 18h; | 100% |
| potassium fluoride on basic alumina for 0.25h; microwave irradiation; | 86% |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
718639-02-2
2-chloro-5-(5-methylfuran-2-yl)pyridine

-
-
718639-13-5
2-{methyl[5-(5-methylfuran-2-yl)pyridin-2-yl]amino}ethanol

| Conditions | Yield |
|---|---|
| for 24h; Heating; | 100% |
-
-
607723-54-6
4-chloro-6-(4-methoxyphenoxy)pyrimidine

-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
607723-69-3
2-{[6-(4-methoxyphenoxy)pyrimidin-4-yl]methylamino}ethanol

| Conditions | Yield |
|---|---|
| In ethanol Heating; | 100% |
| In N,N-dimethyl-formamide at 80℃; Temperature; Solvent; Large scale; | 1.66 kg |
-
-
869639-13-4
4-chloro-6-(3,4-dimethoxyphenoxy)pyrimidine

-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
869639-16-7
2-{[6-(3,4-dimethoxyphenoxy)pyrimidin-4-yl]methylamino}ethanol

| Conditions | Yield |
|---|---|
| In ethanol Heating; | 100% |
-
-
607723-59-1
4-(biphenyl-4-yloxy)-6-chloropyrimidine

-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
607723-75-1
2-{[6-(biphenyl-4-yloxy)pyrimidin-4-yl]methylamino}ethanol

| Conditions | Yield |
|---|---|
| In ethanol Heating; | 100% |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
124041-01-6
4-chloro-6-(4-fluorophenoxy)pyrimidine

-
-
607723-67-1
2-{[6-(4-fluorophenoxy)pyrimidin-4-yl]methylamino}ethanol

| Conditions | Yield |
|---|---|
| In ethanol Heating; | 100% |

-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

| Conditions | Yield |
|---|---|
| In acetonitrile at 25℃; for 4h; | 100% |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

| Conditions | Yield |
|---|---|
| With N-ethylmorpholine;; (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate at 20℃; for 3h; | 100% |
| With N-ethylmorpholine;; (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate at 20℃; for 3h; | 100% |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
55661-08-0
4-methoxy-2,6-dimethylbenzenesulfonyl chloride

-
-
766558-31-0
N-(2-hydroxyethyl)-4-methoxy-2,6,N-trimethylbenzenesulphonamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 3h; | 100% |
| With triethylamine In dichloromethane at 0 - 20℃; for 15h; | 100% |
| With triethylamine In dichloromethane at 20℃; for 15h; | 99% |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
589-15-1
1-bromomethyl-4-bromobenzene

-
-
918642-06-5
2-[(4-bromo-benzyl)-methyl-amino]-ethanol

| Conditions | Yield |
|---|---|
| In 1,2-dimethoxyethane at 40℃; for 1h; | 100% |
| With potassium carbonate In acetonitrile at 20℃; for 2h; |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
1071504-75-0, 1071504-73-8, 1071504-74-9
10,11-dihydro-5H-benzo[e]pyrido[2,3-b]azepine-10-carboxylic acid ethyl ester

-
-
1071504-81-8
N-(2-hydroxyethyl)-N-methyl-10,11-dihydro-5H-benzo[e]pyrido[2,3-b]azepine-10-carboxamide

| Conditions | Yield |
|---|---|
| at 20 - 70℃; for 120.24h; Product distribution / selectivity; | 100% |
| at 20 - 70℃; for 110.25h; Product distribution / selectivity; | 100% |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
62640-03-3
2-(methylamino)ethan-1-ol hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 20℃; for 2h; | 100% |
| With hydrogenchloride In water |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
28188-41-2
3-(bromomethyl)benzonitrile

-
-
1141474-62-5
3-{[(2-hydroxyethyl)methylamino]methyl}benzonitrile

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 0.5h; | 100% |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
1023524-14-2
1-[2-[(chloromethyl)phenyl]ethyl]-3,5,7,9,11,13,15-heptacyclopentylpentacyclo[9.5.1.13,9.15,15.17,13]octasiloxane

-
-
1234502-87-4
C47H81NO13Si8

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane for 24h; Reflux; Inert atmosphere; | 100% |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
2168-84-5
methyl dithioacetate

-
-
69394-45-2
2-hydroxy-N-methylethanethioamide

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0 - 20℃; for 15h; Inert atmosphere; | 100% |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

| Conditions | Yield |
|---|---|
| Stage #1: (4-nitro-1H-pyrazol-5-yl)methanol With carbon tetrabromide; triphenylphosphine In dichloromethane at 0℃; for 1.33333h; Stage #2: (2-hydroxyethyl)(methyl)amine With N-ethyl-N,N-diisopropylamine In dichloromethane at 10℃; for 1h; | 100% |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
1394285-00-7
1,9-dichloro-5-phenyldipyrromethene

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 70℃; for 57h; Inert atmosphere; | 100% |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
140-88-5
ethyl acrylate

-
-
10494-78-7
N-Methyl-N-(2-hydroxyethyl)-β-alanin-ethylester

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; Michael Addition; Inert atmosphere; | 100% |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
876-08-4
p-(chloromethyl)benzoyl chloride

-
-
1182906-72-4
4-(chloromethyl)-N-(2-hydroxyethyl)-N-methylbenzamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 10℃; for 0.166667h; Solvent; Reagent/catalyst; | 100% |
-
-
109-09-1
2-chloropyridine

-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
122321-04-4
2-(N-methyl-N-(pyridin-2-yl)amino)ethanol

| Conditions | Yield |
|---|---|
| for 9h; Reflux; | 99% |
| With triphenylmethyl sodium at 70℃; for 8h; Temperature; | 97% |
| at 140℃; for 0.333333h; Sealed vessel; Neat (no solvent); | 92% |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
171178-25-9
4-(3-bromoanilino)-7-fluoropyrido[4,3-d]pyrimidine

| Conditions | Yield |
|---|---|
| In iso-butanol at 100℃; for 48h; | 99% |

| Conditions | Yield |
|---|---|
| In water at 20℃; for 1h; Condensation; | 99% |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
26452-81-3
4-chloro-6-methoxy-pyrimidine

-
-
869639-14-5
2-[(6-methoxypyrimidin-4-yl)methylamino]ethanol

| Conditions | Yield |
|---|---|
| In ethanol for 24h; Heating; | 99% |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
369-34-6
3,4-difluoronitrobenzene

-
-
677727-07-0
2-[2-Fluoro(methyl)-4-nitroanilino]ethanol

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 100℃; for 1.5h; | 99% |
| With N-ethyl-N,N-diisopropylamine In ethyl acetate at 0 - 20℃; for 24h; | 79% |
2-Methylaminoethanol Chemical Properties
Chemical Name: 2-Methylaminoethanol
IUPAC NAME: 2-(methylamino)ethanol
CAS No.: 109-83-1
Molecular Formula: C3H9NO
Formula Weight: 75.11 g/mol
Structure:
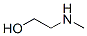
The synonyms of 2-Methylaminoethanol (CAS NO.109-83-1): Methylethanolamine ; N-Methylethanolamine ; N-Methyl(b-hydroxyethyl)mine ; Methylethylolamine ; 2-(Methylamino)ethanol ; (2-Hydroxyethyl)methylamine ; (Hydroxyethyl)methylamine
Polar Surface Area: 32.26 Å2
Index of Refraction: 1.413
Molar Refractivity: 21.07 cm3
Molar Volume: 84.3 cm3
Surface Tension: 29.2 dyne/cm
Enthalpy of Vaporization: 45.68 kJ/mol
Vapour Pressure: 1.1 mmHg at 25°C
Density of 2-Methylaminoethanol (CAS NO.109-83-1): 0.935 g/mL at 25 °C
Boiling Point: 159 °C
Flash Point: 76 °C
Appearance: viscous colourless or light yellow liquid, fishy odor.
Stability: Stable under normal temperatures and pressures. but incompatible with strong oxidizing agents.Combustible.
2-Methylaminoethanol Uses
2-Methylaminoethanol (CAS NO.109-83-1) is used as a chemical intermediate for pharmaceuticals and in textile industry.
2-Methylaminoethanol Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 125mg/kg (125mg/kg) | National Technical Information Service. Vol. AD277-689, | |
| mouse | LD50 | subcutaneous | 1802mg/kg (1802mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES BEHAVIORAL: COMA | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 225, Pg. 428, 1955. |
| rabbit | LD50 | skin | 1070uL/kg (1.07mL/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | National Technical Information Service. Vol. OTS0536158, |
| rat | LD50 | intraperitoneal | 1330mg/kg (1330mg/kg) | Toxicology and Applied Pharmacology. Vol. 12, Pg. 486, 1968. | |
| rat | LD50 | oral | 1391mg/kg (1391mg/kg) | SENSE ORGANS AND SPECIAL SENSES: CHROMODACYRORREA: EYE BEHAVIORAL: ATAXIA GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Veterinary and Human Toxicology. Vol. 38, Pg. 422, 1996. |
2-Methylaminoethanol Consensus Reports
Reported in EPA TSCA Inventory.
2-Methylaminoethanol Safety Profile
Hazard Codes:  C
C
Risk Statements: 21/22-34
R21/22: Harmful in contact with skin and if swallowed.
R34: Causes burns.
Safety Statements: 26-36/37/39-45
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection.
S45: In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
RIDADR: UN 2735 8/PG 2
WGK Germany: 1
RTECS: KL6650000
HazardClass: 8
PackingGroup: III
HS Code: 29221980
Poison by intraperitoneal route. Moderately toxic by ingestion and subcutaneous routes. A corrosive irritant to skin, eyes, and mucous membranes. Flammable when exposed to heat, flame, or oxidizers. To fight fire, use alcohol foam. When heated to decomposition it emits toxic fumes such as NOx. See also AMINES and ALCOHOL, DENATURED; ALCOHOLS, C6-12; ALCOHOLS, C9-11; ALCOHOLS, C12-13, ETHOXYLATED; ALCOHOLS, C12-15, ETHOXYLATED; ALCOHOLS, C12-16, ETHOXYLATED; ALCOHOLS, C14-15, ETHOXYLATED; ALCOHOLS, C16-18, ETHOXYLATED; ALCOHOLS, C8-10, ETHOXYLATED PROPOXYLATED; ALCOHOLS, C12-15, ETHOXYLATED PROPOXYLATED; ALCOHOLS, N.O.S..
2-Methylaminoethanol Specification
Storage: Store in a cool, dry place. Keep away from direct sunlight. Keep container closed when not in use.
Handling: Do not ingest or inhale. Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Use only in a chemical fume hood.
Related Products
- 2-Methylaminoethanol
- 109836-81-9
- 109838-85-9
- 109-84-2
- 1098-45-9
- 109-85-3
- 109857-81-0
- 1098-60-8
- 109-86-4
- 109871-20-7
- 109-87-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View