-
Name
2-Vinylpyridine
- EINECS 202-879-8
- CAS No. 100-69-6
- Article Data103
- CAS DataBase
- Density 0.968 g/cm3
- Solubility 2.5 g/100 mL (20 °C) in water
- Melting Point -50 °C
- Formula C7H7N
- Boiling Point 160.3 °C at 760 mmHg
- Molecular Weight 105.139
- Flash Point 46.7 °C
- Transport Information UN 3073 6.1/PG 2
- Appearance dark brown liquid with an unpleasant odour
- Safety 16-23-26-36/37/39-45-28A
- Risk Codes 10-20-25-34-42/43
-
Molecular Structure
-
Hazard Symbols
 T,
T, C,
C, F
F
- Synonyms Pyridine,2-vinyl- (8CI);2-Ethenylpyridine;2-Pyridylethylene;NSC18255;a-Vinylpyridine;
- PSA 12.89000
- LogP 1.72460
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen In toluene at 20℃; under 22801.5 Torr; for 12h; Autoclave; chemoselective reaction; | 99% |
| With hydrogen In toluene at 110℃; under 760.051 Torr; for 24h; Schlenk technique; | 87% |
| With ammonium formate In N,N-dimethyl-formamide at 80℃; Green chemistry; | 76% |
-
-
25877-30-9
diethyl-(2-pyridin-2-yl-ethyl)-amine

-
-
100-69-6
2-vinylpyridine

| Conditions | Yield |
|---|---|
| 92.3% |

| Conditions | Yield |
|---|---|
| With (IMes)CuCl; triphenylphosphine; isopropyl alcohol In 1,4-dioxane; diethyl ether at 60℃; for 16h; | 86% |

| Conditions | Yield |
|---|---|
| With cesium fluoride In ethanol; water at 50℃; for 5h; Stille coupling; Inert atmosphere; | 82% |
-
-
138428-37-2
2-(pyridin-2-yl)ethyl methanesulfonate

-
-
100-69-6
2-vinylpyridine

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In water at 40℃; for 2h; | 80% |
| With sodium carbonate |

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran for 6h; Ambient temperature; | 73% |

| Conditions | Yield |
|---|---|
| With acetic acid for 12h; Heating; | 70% |
| With sodium tetrafluoroborate; acetic acid In N,N-dimethyl acetamide at 150℃; for 24h; Reagent/catalyst; | 64% |
| With tris(triphenylphosphine)ruthenium(II) chloride; 1,3-bis-(diphenylphosphino)propane; sodium t-butanolate In toluene at 111℃; for 24h; Inert atmosphere; | 41% |

| Conditions | Yield |
|---|---|
| borininato-cobalt-COD catalyst at 120℃; under 38253.1 Torr; for 2h; | 62% |
| With (η-1,5-cyclooctadien(<1-6-η-(1-phenylborinato)>cobalt at 120℃; under 38253.1 Torr; for 2.08333h; | 61.5% |

| Conditions | Yield |
|---|---|
| With sodium tetrafluoroborate; dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; copper diacetate; acetic acid In N,N-dimethyl acetamide at 150℃; for 24h; Time; | A 26% B 53% |


| Conditions | Yield |
|---|---|
| at 800℃; under 0.1 Torr; | 45% |
-
-
82198-70-7
1,2-bis(2-pyridyl)ethane N-oxide

-
A
-
110-86-1
pyridine

-
B
-
100-69-6
2-vinylpyridine

-
C
-
109-06-8
α-picoline

-
D
-
4916-40-9
1,2-bis(pyridin-2-yl)ethane

-
E
-
1437-15-6
1,2-bis(2-pyridyl)ethylene

| Conditions | Yield |
|---|---|
| at 800℃; under 0.05 - 0.2 Torr; Product distribution; Mechanism; | A 11% B 39% C 11% D 7% E 20% |

-
-
56983-98-3
(2-pyridyl)acetaldoxime

-
A
-
110-86-1
pyridine

-
B
-
100-69-6
2-vinylpyridine

-
C
-
109-06-8
α-picoline

-
D
-
100-71-0
2-Ethylpyridine

-
E
-
73177-35-2
2-methyl-1H-pyrrolo<3,2-b>pyridine

-
F
-
4916-40-9
1,2-bis(pyridin-2-yl)ethane

| Conditions | Yield |
|---|---|
| at 800℃; under 0.001 - 0.01 Torr; approximate contact time: 0.002-0.03 s; | A 18% B 5% C 32% D 11% E 4% F 32% |

-
-
109-06-8
α-picoline

-
-
67-56-1
methanol

-
A
-
100-69-6
2-vinylpyridine

-
B
-
108-48-5
2,6-dimethylpyridine

-
C
-
100-71-0
2-Ethylpyridine

-
D
-
108-47-4
2,4-lutidine

| Conditions | Yield |
|---|---|
| Cs exchanged zeolite at 450℃; Product distribution; investigation of the heterogeneous vapor-phase alkylation of α-picoline with methanol over Na+, K+, Rb+, or Cs+ exchanged X- or Y-type zeolite in an atmosphere of nitrogen; | A 3.1% B 7.3% C 30.2% D 3.6% |

-
-
931-19-1
2-methylpyridine N-oxide

-
A
-
110-86-1
pyridine

-
B
-
100-69-6
2-vinylpyridine

-
C
-
109-06-8
α-picoline

-
D
-
1132-37-2
bis(2-pyridyl)methane

-
E
-
4916-40-9
1,2-bis(pyridin-2-yl)ethane

-
F
-
1437-15-6
1,2-bis(2-pyridyl)ethylene

| Conditions | Yield |
|---|---|
| at 800℃; under 0.1 - 1 Torr; Product distribution; Mechanism; other temperatures; other pressure; different carriers; various alkyl derivatives of pyridine N-oxides; | A 24% B 7% C 17% D 8% E 26% F 11% |

-
-
54856-82-5
3-methyl-[1,2,3]triazolo[1,5-a]pyridine

-
-
100-69-6
2-vinylpyridine

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal In ethanol at 150℃; for 24h; Autoclave; | 23% |
-
-
110-86-1
pyridine

-
-
67-56-1
methanol

-
A
-
100-69-6
2-vinylpyridine

-
B
-
109-06-8
α-picoline

-
C
-
108-89-4
picoline

-
D
-
100-71-0
2-Ethylpyridine

-
E
-
536-75-4
4-Ethylpyridine

-
F
-
108-99-6
3-Methylpyridine

| Conditions | Yield |
|---|---|
| Cs exchanged zeolite at 450℃; Product distribution; investigation of the heterogeneous vapor-phase alkylation of pyridine with methanol over Na+, K+, Rb+, or Cs+ exchanged X- or Y-type zeolite in an atmosphere of nitrogen; | A 5.5% B 6.5% C 5% D 22.2% E 5.3% F 3.1% |

-
-
54856-82-5
3-methyl-[1,2,3]triazolo[1,5-a]pyridine

-
-
98-09-9
benzenesulfonyl chloride

-
-
67-64-1
acetone

-
A
-
100-69-6
2-vinylpyridine

-
B
-
10445-92-8
2-(1-chloroethyl)pyridine

-
C
-
127-63-9
diphenyl sulphone

-
D
-
2116-62-3
2-(2-phenylethyl)pyridine

-
E
-
882-33-7
diphenyldisulfane

-
F
-
103-79-7
1-phenyl-acetone

| Conditions | Yield |
|---|---|
| for 80h; Mechanism; Heating; | A n/a B 4% C 6% D n/a E 1% F 3% |

-
-
110-86-1
pyridine

-
-
64-17-5
ethanol

-
A
-
100-69-6
2-vinylpyridine

-
B
-
108-48-5
2,6-dimethylpyridine

-
C
-
536-78-7
3-ethylpyridine

-
D
-
100-71-0
2-Ethylpyridine

-
E
-
536-75-4
4-Ethylpyridine

| Conditions | Yield |
|---|---|
| Cs exchanged zeolite at 450℃; Product distribution; investigation of the heterogeneous vapor-phase alkylation of pyridine with ethanol over Na+, K+, Rb+, or Cs+ exchanged X- or Y-type zeolite in an atmosphere of nitrogen; | A 1.3% B 1.1% C 1.1% D 5.9% E 3.2% |

-
-
931-19-1
2-methylpyridine N-oxide

-
A
-
110-86-1
pyridine

-
B
-
100-69-6
2-vinylpyridine

-
C
-
109-06-8
α-picoline

-
D
-
4916-40-9
1,2-bis(pyridin-2-yl)ethane

| Conditions | Yield |
|---|---|
| at 800℃; under 1 - 5 Torr; | A n/a B 5% C n/a D n/a |


| Conditions | Yield |
|---|---|
| With water at 275℃; beim Leiten ueber mit Zinkfluorid impraegniertes Al2O3; | |
| K-ZSM-5 at 300℃; Conversion of starting material; |

-
-
109-06-8
α-picoline

-
-
50-00-0
formaldehyd

-
A
-
100-69-6
2-vinylpyridine

-
B
-
103-74-2
2-(2-Hydroxyethyl)pyridine

| Conditions | Yield |
|---|---|
| With sulfuric acid; water; hydrogen at 160℃; |


| Conditions | Yield |
|---|---|
| With chromium(III) oxide; iron(III) oxide; potassium hydroxide at 700℃; mit Wasserdampf; | |
| With nitrogen; iodine; oxygen at 700℃; beim Leiten ueber Quarz; | |
| With binary vanadium-magnesium catalyst; water; oxygen at 480℃; Product distribution; oxidative dehydrogenation; other alkylpyridines, also in the presence sulfur dioxide, variously modified catalysts, var. temp.; |
-
-
109-04-6
2-bromo-pyridine

-
-
754-05-2
ethenyltrimethylsilane

-
A
-
100-69-6
2-vinylpyridine

-
B
-
2857-97-8
trimethylsilyl bromide

-
C
-
107-46-0
Hexamethyldisiloxane

-
D
-
106-99-0
buta-1,3-diene

| Conditions | Yield |
|---|---|
| bis(η3-allyl-μ-chloropalladium(II)) In tetralin; water; N,N-dimethyl-formamide at 100℃; for 5h; other catalysts: (Ph3P)4Pd, LiPdCl3, (PhCN)2PdCl2, (MeCN)2PdCl2, Pd/C; Et3N presence; Further byproducts given; | A 30 % Chromat. B n/a C n/a D n/a |

-
-
5029-67-4
2-iodopyridine

-
-
754-05-2
ethenyltrimethylsilane

-
A
-
100-69-6
2-vinylpyridine

-
B
-
16029-98-4
trimethylsilyl iodide

-
C
-
107-46-0
Hexamethyldisiloxane

-
D
-
106-99-0
buta-1,3-diene

| Conditions | Yield |
|---|---|
| bis(η3-allyl-μ-chloropalladium(II)) In tetralin; water; N,N-dimethyl-formamide at 100℃; for 5h; other catalysts: (Ph3P)4Pd, LiPdCl3, (PhCN)2PdCl2, (MeCN)2PdCl2, Pd/C; Et3N presence; Further byproducts given; | A 23 % Chromat. B n/a C n/a D n/a |

-
-
931-19-1
2-methylpyridine N-oxide

-
A
-
110-86-1
pyridine

-
B
-
100-69-6
2-vinylpyridine

-
C
-
109-06-8
α-picoline

-
D
-
1132-37-2
bis(2-pyridyl)methane

-
E
-
4916-40-9
1,2-bis(pyridin-2-yl)ethane

| Conditions | Yield |
|---|---|
| at 550 - 800℃; Product distribution; flash vacuum pyrolysis; |

-
-
931-19-1
2-methylpyridine N-oxide

-
A
-
100-69-6
2-vinylpyridine

-
B
-
100-71-0
2-Ethylpyridine

-
C
-
586-98-1
2-Hydroxymethylpyridine

-
D
-
1132-37-2
bis(2-pyridyl)methane

-
E
-
4916-40-9
1,2-bis(pyridin-2-yl)ethane

-
F
-
1437-15-6
1,2-bis(2-pyridyl)ethylene

| Conditions | Yield |
|---|---|
| at 500 - 800℃; under 0.01 - 0.1 Torr; Product distribution; other pyridine and pyridazine N-oxides; |


| Conditions | Yield |
|---|---|
| With η6-borinato cobalt complexes at 120 - 160℃; under 37503 Torr; Product distribution; |

-
-
110232-64-9
2-[2-([1,3,2]Dioxaphospholan-2-yloxy)-ethyl]-pyridine

-
-
100-69-6
2-vinylpyridine

| Conditions | Yield |
|---|---|
| at 800℃; | |
| at 700℃; under 0.001 Torr; |
-
-
31656-92-5
1-azido-2-methyl-benzene

-
A
-
100-69-6
2-vinylpyridine

-
B
-
16118-22-2
benzylidenamine

-
C
-
64372-87-8
6-Methylene-2,4-cyclohexadien-1-imine

-
D
-
100-47-0
benzonitrile

| Conditions | Yield |
|---|---|
| at 800℃; |


| Conditions | Yield |
|---|---|
| at 60℃; for 2.5h; Alkylation; | 100% |
| With zinc(II) nitrate hexahydrate In acetonitrile at 25℃; for 0.166667h; Sealed tube; Green chemistry; | 92% |
| In water at 20℃; | 84% |
| With benzene | |
| unter Zusatz von wenig wss. Benzyl-trimethyl-ammonium-hydroxid-Loesung auf 100grad; |

| Conditions | Yield |
|---|---|
| With 5% Pd/C; hydrogen In ethanol at 20℃; for 0.5h; chemoselective reaction; | 100% |
| With 6C53H32O8(4-)*13Zr(4+)*18O(2-)*8Co(2+)*8H(1-); hydrogen In tetrahydrofuran at 20℃; under 30003 Torr; for 36h; Catalytic behavior; | 99% |
| With cobalt In tetrahydrofuran Heating; High pressure; chemoselective reaction; | 99% |
-
-
100-69-6
2-vinylpyridine

-
-
956472-60-9
2-(4′-vinylbiphenyl-4-yl)pyridine

| Conditions | Yield |
|---|---|
| Stage #1: 2-vinylpyridine With (diphenylmethyl)potassium Stage #2: 2-(4′-vinylbiphenyl-4-yl)pyridine at -78℃; | 100% |
-
-
100-69-6
2-vinylpyridine

-
-
1632067-27-6
(2-vinylpyridine)Al(C6F5)3

| Conditions | Yield |
|---|---|
| In toluene at 25℃; | 100% |

| Conditions | Yield |
|---|---|
| With oxygen; hydrazine hydrate In acetonitrile at 32℃; under 760.051 Torr; for 3h; Schlenk technique; | 100% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 6h; Green chemistry; regioselective reaction; | 100% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 6h; Green chemistry; regioselective reaction; | 100% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 6h; Green chemistry; regioselective reaction; | 100% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 6h; Green chemistry; regioselective reaction; | 100% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 6h; Green chemistry; regioselective reaction; | 100% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 6h; Green chemistry; regioselective reaction; | 100% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 6h; Green chemistry; regioselective reaction; | 100% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 6h; Green chemistry; regioselective reaction; | 100% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 6h; Green chemistry; regioselective reaction; | 100% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 6h; Green chemistry; regioselective reaction; | 100% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 6h; Green chemistry; regioselective reaction; | 100% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 6h; Green chemistry; regioselective reaction; | 100% |


| Conditions | Yield |
|---|---|
| With potassium phosphate; sodium chloride In water; N,N-dimethyl-formamide at 45℃; for 16h; Catalytic behavior; Reagent/catalyst; Micellar solution; | 99% |
| With potassium carbonate; palladium on activated charcoal In N,N-dimethyl-formamide at 100℃; | 44% |
-
-
100-69-6
2-vinylpyridine

| Conditions | Yield |
|---|---|
| With ethyl 2-methyl-2-methyl tellurium propionate; 2,2'-azobis(isobutyronitrile) at 60℃; for 3h; Product distribution / selectivity; | 99% |
-
-
100-69-6
2-vinylpyridine

-
-
626-55-1
3-Bromopyridine

-
-
13362-75-9
trans-1-(2-pyridyl)-2-(3-pyridyl)ethylene

| Conditions | Yield |
|---|---|
| With dicyclohexyl(2',4',6'-triisopropyl-[1,1':3',1''-terphenyl]-2-yl)phosphane; palladium diacetate; sodium hydrogencarbonate In N,N-dimethyl-formamide at 120℃; for 8h; Sealed tube; Inert atmosphere; Glovebox; Schlenk technique; | 99% |
| With monophosphine 1,2,3,4,5-pentaphenyl-1'-(di-tert-butylphosphino)ferrocene; palladium diacetate; sodium hydrogencarbonate In N,N-dimethyl-formamide at 120℃; for 4h; Reagent/catalyst; Heck Reaction; Inert atmosphere; | 99% |
| With C18H21N3O2Pd; tetrabutylammomium bromide; caesium carbonate In N,N-dimethyl acetamide at 150℃; for 15h; Heck Reaction; Sealed tube; | 60% |
-
-
100-69-6
2-vinylpyridine

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol for 36h; Reflux; | 99% |
-
-
100-69-6
2-vinylpyridine

-
-
23516-84-9
2,2,2-trifluoro-1-(4-iodophenyl)ethan-1-one

| Conditions | Yield |
|---|---|
| With diphenyl hydrogen phosphate; diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate In dichloromethane at 20℃; Irradiation; Inert atmosphere; | 99% |
-
-
100-69-6
2-vinylpyridine

-
-
4559-70-0
Diphenylphosphine oxide

-
-
25898-97-9
α-<2-(diphenylphosphinyl)ethyl>pyridine

| Conditions | Yield |
|---|---|
| With pyridine; lanthanum tris(N,N-dimethylbenzylamine) at 80℃; for 16h; Glovebox; regioselective reaction; | 98% |
| at 120℃; for 1h; microwave irradiation; | 88% |
| With silver fluoride In N,N-dimethyl-formamide at 110℃; for 24h; | 80% |
| In benzene at 140℃; for 3.5h; | 78% |
| With oxygen at 80℃; for 12h; | 95 % Spectr. |

| Conditions | Yield |
|---|---|
| at 60℃; for 2.5h; Alkylation; | 98% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 40℃; for 0.5h; | 98% |
| at 60℃; for 2.5h; Alkylation; | 93% |
-
-
100-69-6
2-vinylpyridine

-
-
75-15-0
carbon disulfide

-
-
100-46-9
benzylamine

-
-
21172-92-9
2-(pyridin-2-yl)ethyl benzylcarbamodithioate

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 6h; Green chemistry; regioselective reaction; | 98% |


| Conditions | Yield |
|---|---|
| In chloroform Inert atmosphere; Schlenk technique; | 98% |
-
-
100-69-6
2-vinylpyridine

-
-
254454-54-1
tert-butyl-3-iodoazetidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In water; acetonitrile at 20℃; for 16h; Irradiation; chemoselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| With (dpp-BIAN)Mg(THF)3 In benzene-d6 at 20℃; for 4.1h; Reagent/catalyst; Time; Inert atmosphere; Schlenk technique; | 97% |
| With NPs-Fe3O4-DE-bmim3PW In dichloromethane at 20℃; for 2h; | 90% |
| With ferrocene; C42H48N2Si2Y(1-)*C16H32LiO4(1+) In benzene-d6 at 130℃; for 0.08h; Inert atmosphere; Glovebox; | 84% |
2-Vinylpyridine Consensus Reports
2-Vinylpyridine Specification
The 2-Vinylpyridine, with the CAS registry number 100-69-6, is also known as 2VP. It belongs to the product categories of Pyridines Derivates; Monomers; Polymer Science; Vinyl Halides, Amines, Amides, and Other Vinyl Monomers; C7 and C8; Heterocyclic Building Blocks; Pyridines. Its EINECS registry number is 202-879-8. This chemical's molecular formula is C7H7N and molecular weight is 105.13718. Its IUPAC name is called 2-ethenylpyridine. The product can be used to prepare modification latex of vinyl pyridine with butadiene or styrene copolymerization. In addition, it also can be used for binder. 2-Vinylpyridine is a useful intermediate for the production of polymers.
Physical properties of 2-Vinylpyridine: (1)ACD/LogP: 1.34; (2)ACD/LogD (pH 5.5): 1.23; (3)ACD/LogD (pH 7.4): 1.34; (4)ACD/BCF (pH 5.5): 4.78; (5)ACD/BCF (pH 7.4): 6.1; (6)ACD/KOC (pH 5.5): 99.31; (7)ACD/KOC (pH 7.4): 126.9; (8)#H bond acceptors: 1; (9)#Freely Rotating Bonds: 1; (10)Index of Refraction: 1.562; (11)Molar Refractivity: 35.26 cm3; (12)Molar Volume: 108.6 cm3; (13)Surface Tension: 36.1 dyne/cm; (14)Density: 0.968 g/cm3; (15)Flash Point: 46.7 °C; (16)Enthalpy of Vaporization: 38.05 kJ/mol; (17)Boiling Point: 160.3 °C at 760 mmHg; (18)Vapour Pressure: 3.13 mmHg at 25°C.
Preparation of 2-Vinylpyridine: this chemical can be obtained from 2-hydroxyethyl pyridine by dehydration.

Uses of 2-Vinylpyridine: it can be used to produce 4-chlorophenyl 2-(2-pyridyl)ethyl sulfide at temperature of 60 °C. This reaction is a kind of Alkylation. It will need 2.5 hours. The yield is about 93%.
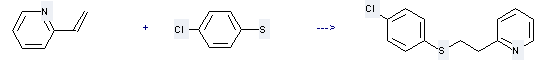
When you are using this chemical, please be cautious about it as the following:
This chemical may destroy living tissue on contact and may cause burns. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective clothing, gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: C=CC1=CC=CC=N1
(2)InChI: InChI=1S/C7H7N/c1-2-7-5-3-4-6-8-7/h2-6H,1H2
(3)InChIKey: KGIGUEBEKRSTEW-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LDLo | skin | 500mg/kg (500mg/kg) | "Prehled Prumyslove Toxikologie; Organicke Latky," Marhold, J., Prague, Czechoslovakia, Avicenum, 1986Vol. -, Pg. 845, 1986. | |
| mammal (species unspecified) | LD50 | oral | 415mg/kg (415mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 39(4), Pg. 86, 1974. | |
| mouse | LC50 | inhalation | 460mg/m3 (460mg/m3) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 10(3), Pg. 9, 1966. | |
| mouse | LD50 | oral | 420mg/kg (420mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 10(3), Pg. 9, 1966. | |
| rat | LC50 | inhalation | 610mg/m3 (610mg/m3) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 57(9-10), Pg. 64, 1992. | |
| rat | LD50 | oral | 100mg/kg (100mg/kg) | "Prehled Prumyslove Toxikologie; Organicke Latky," Marhold, J., Prague, Czechoslovakia, Avicenum, 1986Vol. -, Pg. 845, 1986. |
Related Products
- 2-Vinylpyridine
- 1006-99-1
- 1007-01-8
- 1007-03-0
- 100704-10-7
- 1007-06-3
- 100707-39-9
- 1007089-84-0
- 100-70-9
- 10070-92-5
- 100-71-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View