-
Name
3-Chlorophenol
- EINECS 203-582-6
- CAS No. 108-43-0
- Article Data192
- CAS DataBase
- Density 1.218 g/cm3
- Solubility 27.7 g/L (20 °C) in water
- Melting Point 31-34 °C(lit.)
- Formula C6H5ClO
- Boiling Point 214 °C at 760 mmHg
- Molecular Weight 128.558
- Flash Point 76.9 °C
- Transport Information UN 2020 6.1/PG 3
- Appearance White to light yellow crystal
- Safety 28-61-28A
- Risk Codes 20/21/22-51/53
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, N
N
- Synonyms Phenol,m-chloro- (7CI,8CI);3-Hydroxychlorobenzene;NSC 59700;m-Chlorophenic acid;m-Chlorophenol;
- PSA 20.23000
- LogP 2.04560
Synthetic route
-
-
123195-73-3
1-tert-butoxy-3-chlorobenzene

-
-
108-43-0
3-monochlorophenol

| Conditions | Yield |
|---|---|
| With sodium iodide; cerium(III) chloride In acetonitrile at 40℃; for 3.5h; | 100% |
-
-
24318-02-3
1-(benzyloxy)-3-chlorobenzene

-
-
108-43-0
3-monochlorophenol

| Conditions | Yield |
|---|---|
| With 1-n-butyl-3-methylimidazolim bromide at 200 - 220℃; for 0.0833333h; Microwave irradiation; Inert atmosphere; | 99% |
| With triethylsilane; triethylamine; palladium diacetate In dichloromethane at 23℃; for 12h; Hydrogenolysis; |

| Conditions | Yield |
|---|---|
| With 9,10-dihydroanthracene; water at 356.85℃; for 3h; Kinetics; | A 99% B 0.3% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; tris-(dibenzylideneacetone)dipalladium(0); tert-butyl XPhos In 1,4-dioxane; water at 100℃; for 1h; | 99% |
| Multi-step reaction with 2 steps 1: copper dichloride; potassium carbonate / 20 h / 130 °C / Inert atmosphere; Schlenk technique 2: potassium hydroxide / dimethyl sulfoxide / 3 h / 100 °C / Schlenk technique View Scheme | |
| Multi-step reaction with 2 steps 1: fac-tris(2-phenylpyridinato-N,C2')iridium(III); tributyl-amine; water / acetonitrile / 36 h / 25 °C / Schlenk technique; Inert atmosphere; Sealed tube; Irradiation 2: air / 16 h / 25 °C / Inert atmosphere View Scheme | |
| With copper(l) iodide; C20H20N4O4; caesium carbonate; Benzaldoxime In dimethyl sulfoxide at 80℃; for 18h; Inert atmosphere; Glovebox; |
-
-
63503-60-6
3-chlorophenylboronic acid

-
-
108-43-0
3-monochlorophenol

| Conditions | Yield |
|---|---|
| With water In tetrahydrofuran at 100℃; for 12h; | 99% |
| With copper(I) oxide; potassium hydroxide In water at 20℃; for 24h; Green chemistry; | 98% |
| With copper(II) ferrite; water; sodium hydroxide at 40℃; for 24h; Green chemistry; | 96% |

| Conditions | Yield |
|---|---|
| With 1-n-butyl-3-methylimidazolim bromide at 200 - 220℃; for 0.666667h; Microwave irradiation; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 100℃; for 3h; Schlenk technique; | 99% |

| Conditions | Yield |
|---|---|
| With glycolic Acid; copper hydroxide; sodium hydroxide In water; dimethyl sulfoxide at 120℃; for 6h; Inert atmosphere; Schlenk technique; | 98% |
| With copper acetylacetonate; N1-(4-hydroxy-2,6-dimethylphenyl)-N2-(4-hydroxy-3,5-dimethylphenyl)oxalamide; potassium hydroxide In water; dimethyl sulfoxide at 60℃; for 24h; Schlenk technique; Inert atmosphere; chemoselective reaction; | 95% |
| With copper(I) oxide; 2-(N,N-dimethylamino)ethanol; water; potassium hydroxide In dimethyl sulfoxide at 110℃; for 24h; Inert atmosphere; | 91% |

| Conditions | Yield |
|---|---|
| With ammonium bicarbonate In water at 20℃; for 2h; Schlenk technique; | 97% |

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; (1,2-dimethoxyethane)dichloronickel(II); water; bis(pinacol)diborane; lithium tert-butoxide In methanol; N,N-dimethyl acetamide at 30℃; for 24h; Schlenk technique; | 92% |
| With 6,6'-dimethyl-2,2'-bipyridine; (1,2-dimethoxyethane)dichloronickel(II); bis(pinacol)diborane; lithium tert-butoxide In methanol; N,N-dimethyl acetamide; water at 30℃; for 24h; Inert atmosphere; Glovebox; Sealed tube; | 92% |

-
-
108-43-0
3-monochlorophenol

| Conditions | Yield |
|---|---|
| With tert.-butyl lithium In tetrahydrofuran; pentane at -78℃; for 0.5h; | 91% |
-
-
86992-79-2, 98575-77-0, 65986-73-4
(1S,2S)-3-Chloro-cyclohexa-3,5-diene-1,2-diol

-
A
-
108-43-0
3-monochlorophenol

-
B
-
95-57-8
2-monochlorophenol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 25℃; Kinetics; Concentration; Solvent; | A 89% B n/a |
-
-
159390-33-7
3-chlorophenyl N,N-diethylcarbamate

-
-
108-43-0
3-monochlorophenol

| Conditions | Yield |
|---|---|
| With zirconocene dichloride In tetrahydrofuran at 20℃; Inert atmosphere; | 88% |

| Conditions | Yield |
|---|---|
| 85% |

| Conditions | Yield |
|---|---|
| With [Ni(N,N-bis(4-methoxy-3,5-dimethylpyridin-2-ylmethyl)-N',N'-dimethylpropane-1,3-diamine)(CH3CN)2](Ph4B)2; dihydrogen peroxide; triethylamine In water; acetonitrile at 60℃; for 5h; | A 16% B 84% |
| With C25H29N7O*Fe(2+)*2CF3O3S(1-); dihydrogen peroxide | |
| With dihydrogen peroxide; C23H37ClCuN4O6(1+)*ClO4(1-); triethylamine In acetonitrile at 60℃; for 5h; |

| Conditions | Yield |
|---|---|
| Stage #1: m-Chlorobenzaldehyde With 3-chloro-benzenecarboperoxoic acid for 0.0833333h; Dakin oxidation; Stage #2: With sodium hydroxide for 0.0833333h; Further stages.; | 80% |
-
-
1314570-58-5
1-(but-3-enyloxy)-3-chlorobenzene

-
-
108-43-0
3-monochlorophenol

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; (1,2-dimethoxyethane)dichloronickel(II); water; bis(pinacol)diborane; lithium tert-butoxide In methanol; N,N-dimethyl acetamide at 30℃; for 24h; Schlenk technique; | 75% |
| With 6,6'-dimethyl-2,2'-bipyridine; (1,2-dimethoxyethane)dichloronickel(II); bis(pinacol)diborane; lithium tert-butoxide In methanol; N,N-dimethyl acetamide; water at 30℃; for 24h; Inert atmosphere; Glovebox; Sealed tube; | 75% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-chloro-aniline With sodium nitrite In water at 20℃; Milling; Green chemistry; Stage #2: With water at 85℃; for 0.5h; Neutral conditions; Green chemistry; | 73% |
| With uranyl nitrate hydrate; water; trifluoroacetic acid for 48h; Irradiation; | 56% |
-
-
54058-00-3
diethyl phosphite potassium salt

-
-
86364-03-6
3-chloro-1-trifluoromethanesulfonyloxybenzene

-
A
-
108-43-0
3-monochlorophenol

-
B
-
32019-36-6
3-chlorophenyl diethyl phosphate

| Conditions | Yield |
|---|---|
| In ammonia at -33℃; under 2.5 Torr; | A 30% B 67% |


| Conditions | Yield |
|---|---|
| With 9,10-dihydroanthracene; water at 356.85℃; for 3h; Kinetics; | A 62% B 0.3% |
-
-
19514-06-8
α-(3-Chlorophenoxy)acetophenone

-
A
-
108-43-0
3-monochlorophenol

-
B
-
50781-28-7
2-oxo-2-phenyl-ethyl

-
C
-
27798-43-2
2-(3-chlorophenyl)-1-phenylethanone

-
D
-
54560-44-0
3-chlorophenoxyl radical

-
E
-
98-86-2
acetophenone

| Conditions | Yield |
|---|---|
| thiophenol In benzene Product distribution; Quantum yield; Kinetics; Irradiation; | A 34.2% B n/a C 42.1% D n/a E 23.8% |

-
-
108-90-7
chlorobenzene

-
A
-
108-43-0
3-monochlorophenol

-
B
-
95-57-8
2-monochlorophenol

-
C
-
106-48-9
4-chloro-phenol

-
D
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| With KCu(III)(biuret)2; water; trifluoroacetic acid for 2h; Product distribution; Heating; | A n/a B 6% C 7% D 33% |
| With tert.-butylhydroperoxide; air In gas at 290℃; under 760 Torr; Product distribution; Mechanism; | |
| In tert-butyl alcohol at 488 - 587℃; under 760 Torr; Mechanism; Slow combustion of dichlorobenzene has been studied at low degrees of conversion at low degrees of conversion, in athmospheric pressure, between 410-1053 K , in various solvents.; | |
| cyclohexa-1,3-diene at 570 - 622℃; under 760 Torr; Mechanism; | |
| With air at 686.85℃; Formation of xenobiotics; Further byproducts given. Title compound not separated from byproducts; |

-
-
17333-84-5
3-chlorobenzenediazonium

-
-
108-43-0
3-monochlorophenol

| Conditions | Yield |
|---|---|
| With sulfuric acid Diazotization.Behandlung der Diazoniumsalz-Loesung mit CuOH; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 260℃; | |
| With sodium hydroxide at 200℃; | |
| With methanol; sodium methylate at 180℃; | |
| With sodium methylate; sodium thiomethoxide 1.) HMPA, 120 degC, 2h; 2.) 120 degC, 1h; Yield given. Multistep reaction; |
-
-
75-89-8
2,2,2-trifluoroethanol

-
A
-
108-43-0
3-monochlorophenol

-
B
-
109629-96-1
(2,2,2-trifluoroethoxy)dimethylphenylsilane

| Conditions | Yield |
|---|---|
| With potassium 2,2,2-trifluoroethoxide at 30℃; Rate constant; μ = 0.05 M with potassium trifluoroacetate; |

-
-
2985-80-0
4-chloro-2-hydroxybenzophenone

-
A
-
108-43-0
3-monochlorophenol

-
B
-
13189-55-4
3-chlorophenyl benzoate

| Conditions | Yield |
|---|---|
| trifluorormethanesulfonic acid In 1,2-dichloro-ethane at 170℃; for 24h; Mechanism; Product distribution; in dependence on concentration of TFMS, time; | A 5.1 % Chromat. B 47.7 % Chromat. |
-
-
108-41-8
1-chloro-3-methylbenzene

-
A
-
108-43-0
3-monochlorophenol

-
B
-
873-63-2
m-chlorobenzyl alcohol

-
C
-
587-04-2
3-Chlorobenzaldehyde

-
D
-
535-80-8
3-chlorobenzoate

| Conditions | Yield |
|---|---|
| With water; iron; trifluoroacetic acid In acetone for 1h; Rate constant; | |
| With water; iron; trifluoroacetic acid In pyridine for 1h; Rate constant; | |
| With water; copper; trifluoroacetic acid In pyridine for 1h; Rate constant; | |
| With water; copper; trifluoroacetic acid In acetone for 1h; Rate constant; |

-
-
13031-39-5
3-chlorophenyl acetate

-
-
108-43-0
3-monochlorophenol

| Conditions | Yield |
|---|---|
| With N-butylamine; Tetraethylene glycol dimethyl ether In chlorobenzene at 25℃; Rate constant; efficacy of glyme catalysis, other glymes; | |
| With potassium chloride; poly(ethyleneimine) In water at 25℃; Rate constant; Mechanism; Product distribution; various pH, aminolysis; |
-
-
108-43-0
3-monochlorophenol

-
-
6191-99-7, 99471-66-6
(2E)-3-ethoxyprop-2-enoyl chloride

-
-
105786-77-4
3-Ethoxyacrylsaeure-3-chlorphenylester

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-ethane for 14h; Heating; | 100% |
-
-
108-43-0
3-monochlorophenol

-
-
106-96-7
propargyl bromide

-
-
33302-52-2
1-chloro-3-(prop-2-yn-1-yloxy)benzene

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Reflux; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide; toluene at 60℃; for 16h; Sealed tube; Inert atmosphere; | 95% |
| With potassium carbonate In acetone at 56℃; | 93% |
-
-
108-43-0
3-monochlorophenol

-
-
88-10-8
N,N-diethylcarbamyl chloride

-
-
159390-33-7
3-chlorophenyl N,N-diethylcarbamate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 70℃; for 13.5h; | 100% |
| Stage #1: 3-monochlorophenol With sodium hydride In tetrahydrofuran; mineral oil at 23℃; for 1h; Inert atmosphere; Stage #2: N,N-diethylcarbamyl chloride In tetrahydrofuran; mineral oil at 23℃; for 16h; Inert atmosphere; | 94% |
| Stage #1: 3-monochlorophenol With sodium hydride In tetrahydrofuran for 1.16667h; Stage #2: N,N-diethylcarbamyl chloride In tetrahydrofuran at 20℃; for 14h; | 93% |
-
-
108-43-0
3-monochlorophenol

-
-
350-30-1
3-chloro-4-fluoronitrobenzene

-
-
500540-67-0
2-chloro-1-(3-chlorophenoxy)-4-nitrobenzene

| Conditions | Yield |
|---|---|
| Stage #1: 3-monochlorophenol; 3-chloro-4-fluoronitrobenzene With caesium carbonate In N,N-dimethyl-formamide; acetonitrile at 20℃; Stage #2: With water In N,N-dimethyl-formamide; acetonitrile | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 2h; |
-
-
108-43-0
3-monochlorophenol

-
-
1417617-96-9
benzyl 3-[4-(5-methanesulfonyloxazolo[5,4-d]pyrimidin-2-yl)-2,6-dimethylphenoxy]cyclobutanecarboxylate

-
-
1417617-97-0
benzyl 3-{4-[5-(3-chlorophenoxy)oxazolo[5,4-d]pyrimidin-2-yl]-2,6-dimethylphenoxy}cyclobutanecarboxylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20 - 60℃; for 5.5h; | 100% |
-
-
108-43-0
3-monochlorophenol

-
-
350-46-9
4-Fluoronitrobenzene

-
-
2303-23-3
1-chloro-3-(4-nitrophenoxy)benzene

| Conditions | Yield |
|---|---|
| With cesium fluoride/clinoptilolite In dimethyl sulfoxide at 110℃; for 0.15h; Ullmann Condensation; | 100% |
| With potassium carbonate In dimethyl sulfoxide at 70℃; | 92% |
| With potassium carbonate In acetonitrile at 90℃; for 18h; |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 1.5h; Inert atmosphere; | 100% |
-
-
108-43-0
3-monochlorophenol

| Conditions | Yield |
|---|---|
| With caesium carbonate In acetonitrile at 0 - 20℃; | 100% |
-
-
108-43-0
3-monochlorophenol

-
-
31250-08-5
2-chloroethyl cyanomethyl ether

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl acetamide at 120℃; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With hydrogen; sodium hydroxide In water at 25℃; for 2h; | 99.9% |
| With hydrogen; sodium hydroxide In water at 20℃; under 760.051 Torr; for 1h; | 99.9% |
| With hydrogen; sodium hydroxide In water at 20℃; under 760.051 Torr; for 1.5h; | 99.8% |
-
-
108-43-0
3-monochlorophenol

-
-
40979-03-1
2,4,6-tribromo-3-chlorophenol

| Conditions | Yield |
|---|---|
| With benzyltrimethylammonium tribromide; calcium carbonate In methanol; dichloromethane | 99% |
| With water; potassium bromide |

| Conditions | Yield |
|---|---|
| With Zn(N4,N4'-di(pyridin-4-yl)biphenyl-4,4'-dicarboxamide)(5-aminoisophthalate) In dichloromethane at 20℃; for 14h; | 99% |
| With sulfuric acid at 20℃; for 17h; | 99% |
| With C36H30N4O4*2C3H7NO*2NO3(1-)*Zn(2+)*2C2H3N In dichloromethane at 20℃; for 11h; | 97% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 4h; Reflux; | 99% |
| With potassium carbonate In acetone for 20h; Reflux; | 98% |
| With hydrogenchloride; potassium carbonate In hexane; ethyl acetate; acetone | 58 g (87%) |
-
-
108-43-0
3-monochlorophenol

-
-
15729-19-8
3-(diphenylphosphinyl)-3-methyl-1,2-butadiene

-
-
1176335-48-0
C22H20ClO2P

| Conditions | Yield |
|---|---|
| With (R)-((4,4’-bi-1,3-benzodioxole)-5,5’-diyl)bis(bis(3,5-di-t-butyl-4-methoxyphenyl))phosphine; [Rh(OH)(cod)]2 In tert-butyl alcohol at 80℃; for 24h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 99% |
-
-
299176-17-3
N-(2-nitro-5-chlorophenyl)-N-methylcarbamic acid t-butyl ester

-
-
108-43-0
3-monochlorophenol

-
-
1072002-99-3
tert-butyl [5-(3-chlorophenoxy)-2-nitrophenyl]methylcarbamate

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl acetamide at 80℃; Cooling with ice; | 99% |
| With sodium hydride In N,N-dimethyl-formamide at 80℃; for 10h; Inert atmosphere; | 99% |
-
-
108-43-0
3-monochlorophenol

-
-
122-60-1
Phenyl glycidyl ether

-
-
1260374-17-1
1-(3'-chlorophenoxy)-3-phenoxypropan-2-ol

| Conditions | Yield |
|---|---|
| With 2-tert-butylimino-2-diethylamino-1,3-dimethylperhydro-1,3,2-diazaphosphorine supported on polystyrene (PS-BEMP; PS = 200-400 mesh polystyryl with 2percent of divinylbenzene) at 60℃; for 40h; Neat (no solvent); regioselective reaction; | 99% |
-
-
108-43-0
3-monochlorophenol

-
-
331-64-6
2-fluoro-4-methoxy-benzaldehyde

-
-
1446253-88-8
2-(3-chlorophenoxy)-4-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 120℃; for 3h; Inert atmosphere; | 99% |
-
-
108-43-0
3-monochlorophenol

-
-
1194-02-1
4-fluorobenzonitrile

-
-
1145-58-0
4-(3-chlorophenoxy)benzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-monochlorophenol; 4-fluorobenzonitrile With potassium hydroxide In N,N-dimethyl-formamide at 175℃; for 0.333333h; Sealed tube; Stage #2: With potassium hydroxide In water for 12h; Reflux; | 99% |
| Stage #1: 3-monochlorophenol; 4-fluorobenzonitrile With potassium hydroxide In N,N-dimethyl-formamide at 175℃; for 0.333333h; Sealed tube; Stage #2: With water; potassium hydroxide for 12h; Reflux; | 99% |
-
-
108-43-0
3-monochlorophenol

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; for 18h; Temperature; | 99% |
-
-
108-43-0
3-monochlorophenol

-
-
23579-79-5
3,5-dibromo-1-methyl-1H-1,2,4-triazole

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; for 15h; Sealed tube; | 99% |
3-CHLOROPHENOL Chemical Properties
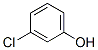
IUPAC:3-chlorophenol
CAS:108-43-0
The Molecular formula of 3-Chlorophenol(108-43-0):C6H5ClO
The Molecular Weight of 3-Chlorophenol(108-43-0):128.56
Synonyms:3-chloro-1-hydroxybenzene;3-chloro-pheno;3-Hydroxychlorobenzene;m-chlorophenicacid;m-chloro-pheno;meta-Chlorophenol;Phenol, m-chloro-;Phenol
EINECS:203-582-6
Density:1.218 g/cm3
Melting Point:33-35oC
Boiling Point:214oC
Flash Point:97oC
Refractive index:n20/D 1.563(lit.)
Water Solubility:27.7 g/L (20oC)
Storage temp.:2-8oC
Stability:Stable.Combustible.Incompatible with acid chlorides,oxidizing agents,acid anhydrides.Discolours in air.
Vapour Pressure:0.109 mmHg at 25oC
Appearance:white crystalline solid with an unpleasant smell
Product Categories:Organics;Phenol&Thiophenol&Mercaptan;Phenoles and thiophenoles;Chlorine Compounds;Phenols;Alpha sort;C;CAlphabetic;CH;Pesticides&Metabolites;Organic Building Blocks;Oxygen Compounds
Mol File:108-43-0.mol
3-CHLOROPHENOL Uses
3-CHLOROPHENOL Toxicity Data With Reference
| 1. | mmo-sat 10 µg/plate | TECSDY Toxicological and Environmental Chemistry. 14 (1987),143. | ||
| 2. | orl-rat LD50:570 mg/kg | FEPRA7 Federation Proceedings, Federation of American Societies for Experimental Biology. 2 (1943),76. | ||
| 3. | ipr-rat LD50:355 mg/kg | BJPCAL British Journal of Pharmacology and Chemotherapy. 13 (1958),20. | ||
| 4. | scu-rat LD50:1390 mg/kg | FEPRA7 Federation Proceedings, Federation of American Societies for Experimental Biology. 2 (1943),76. | ||
| 5. | orl-mus LD50:521 mg/kg | TOLED5 Toxicology Letters. 29 (1985),39. |
3-CHLOROPHENOL Consensus Reports
3-CHLOROPHENOL Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion and subcutaneous routes. Questionable carcinogen with experimental tumorigenic data by skin contact. Mutation data reported. Flammable or combustible liquid. When heated to decomposition it emits toxic fumes of Cl−. See also CHLOROPHENOLS.
Safty informations about 3-Chlorophenol(108-43-0):
Hazard Codes:Xn,N
Risk Statements:20/21/22-51/53
20/21/22(Harmful by inhalation, in contact with skin and if swallowed)
51/53(Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment)
Safety Statements:28-61
28( After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer))
61(Avoid release to the environment. Refer to special instructions safety data sheet)
RIDADR:UN 2020 6.1/PG 3
WGK Germany:2
RTECS:SK2450000
F:9
HazardClass:6.1
HS Code:29081000
3-CHLOROPHENOL Specification
Storage:Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Store protected from moisture.
Handling:Wash thoroughly after handling. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Related Products
- 3-CHLOROPHENOL
- 1084334-28-0
- 108433-95-0
- 108433-99-4
- 108436-80-2
- 108437-64-5
- 108437-87-2
- 108437-88-3
- 108437-89-4
- 108-44-1
- 108444-56-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View