-
Name
3-Chlorobenzonitrile
- EINECS 212-172-6
- CAS No. 766-84-7
- Article Data150
- CAS DataBase
- Density 1.23 g/cm3
- Solubility Insoluble in water
- Melting Point 38-42 °C
- Formula C7H4ClN
- Boiling Point 203.4 °C at 760 mmHg
- Molecular Weight 137.568
- Flash Point 97.2
- Transport Information
- Appearance slightly beige to light pink crystalline powder
- Safety 23-24/25
- Risk Codes 36-21/22
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms Benzonitrile,m-chloro- (7CI,8CI);NSC 59733;m-Chlorobenzonitrile;m-Chlorocyanobenzene;
- PSA 23.79000
- LogP 2.21168
Synthetic route
-
-
150255-96-2
(3‐cyanophenyl)boronic acid

-
-
766-84-7
3-chloro-benzonitrile

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; copper(l) chloride In acetonitrile at 80℃; for 1h; | 98% |
| With N-chloro-succinimide In acetonitrile at 80℃; for 12h; | 97% |

-
-
766-84-7
3-chloro-benzonitrile

| Conditions | Yield |
|---|---|
| With iron(III) chloride; 2,6-di-tert-butyl-4-methyl-phenol In dichloromethane; toluene at 20℃; for 0.0833333h; | 97% |
| With iron(III) chloride; 2,6-di-tert-butyl-4-methyl-phenol In toluene at 20℃; for 0.0833333h; Schlenk technique; | 85% |

| Conditions | Yield |
|---|---|
| With copper(II) oxide; hydroxylamine hydrochloride In neat (no solvent) for 0.0263889h; Mechanism; Reagent/catalyst; Microwave irradiation; Green chemistry; | 95% |
| With ammonium acetate; phenyltrimethylammonium tribromide In acetonitrile at 20℃; for 64h; | 94% |
| Stage #1: m-Chlorobenzaldehyde With ammonia In water at 20℃; for 0.166667h; Stage #2: With tetra-N-butylammonium tribromide In water at 20℃; for 3h; | 93% |

| Conditions | Yield |
|---|---|
| With dmap; copper(l) iodide; 9-azabicyclo[3.3.1]nonane N-oxyl; oxygen; 4,4'-di-tert-butyl-2,2'-bipyridine In acetonitrile at 20℃; under 760.051 Torr; for 15h; Reagent/catalyst; | 95% |
| With 1-methyl-1H-imidazole; oxygen; copper(ll) bromide In dimethyl sulfoxide at 100℃; for 24h; | 90% |
| With potassium hydroxide; nickel copper formate; (Bu4N)2S2O8 In dichloromethane at 20℃; for 10h; Oxidation; | 87% |
-
-
34158-71-9
3-chlorobenzaldehyde oxime

-
-
766-84-7
3-chloro-benzonitrile

| Conditions | Yield |
|---|---|
| With acetonitrile for 1.5h; Reflux; Green chemistry; | 95% |
| Stage #1: 3-chlorobenzaldehyde oxime With Bromotrichloromethane; triphenylphosphine In dichloromethane for 0.416667h; Reflux; Stage #2: With triethylamine In dichloromethane for 8h; Reflux; | 81% |
| With 1,2-bis(3-fluorophenyl)diselane; dihydrogen peroxide In acetonitrile at 65℃; for 24h; | 80% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 120℃; for 15h; Schlenk technique; Green chemistry; | 94% |
| With potassium carbonate In N,N-dimethyl-formamide at 120℃; for 13h; | 88% |
| With sodium carbonate In N,N-dimethyl-formamide at 120℃; for 1h; Inert atmosphere; Green chemistry; | |
| With sodium carbonate In N,N-dimethyl-formamide at 120℃; |

| Conditions | Yield |
|---|---|
| With [Pd{C6H3(CH2CH2NH2)-4-OMe-5-κ2-C,N}(μ-Br)]2; potassium carbonate In N,N-dimethyl-formamide at 130℃; for 0.05h; Microwave irradiation; chemoselective reaction; | 93% |
| With tri-tert-butyl phosphine; potassium carbonate In N,N-dimethyl-formamide at 120℃; for 17h; Inert atmosphere; | 69 %Chromat. |
| With sodium carbonate In N,N-dimethyl-formamide at 120℃; for 1h; Inert atmosphere; Green chemistry; | |
| With sodium carbonate In N,N-dimethyl-formamide at 120℃; |

| Conditions | Yield |
|---|---|
| With tri-tert-butyl phosphine; tributyltin chloride; tris(dibenzylideneacetone)dipalladium (0) In acetonitrile at 80℃; for 17h; | 92% |

| Conditions | Yield |
|---|---|
| With CHF3O3S*C52H45NO2P2Pd; potassium carbonate In N,N-dimethyl-formamide at 130℃; for 3.25h; chemoselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| With sodium carbonate; potassium ferrocyanide In N,N-dimethyl-formamide at 120℃; for 6h; | 91% |
-
-
63503-60-6
3-chlorophenylboronic acid

-
A
-
766-84-7
3-chloro-benzonitrile

-
B
-
6295-97-2
4-bromo-benzenesulfonic acid-(4-chloro-anilide)

| Conditions | Yield |
|---|---|
| With Fe3O4/SiO2/(3-chloropropyl)trimethoxysilane/2,2′-(4,4′-(propane-1,3-diyl)bis(piperazine-4,1-diyl))- diethanamine/Pd In acetonitrile for 15h; Catalytic behavior; Reflux; | A 91% B n/a |

-
-
63503-60-6
3-chlorophenylboronic acid

-
A
-
766-84-7
3-chloro-benzonitrile

-
B
-
16937-03-4
4-nitro-N-(4-chlorophenyl)benzenesulfonamide

| Conditions | Yield |
|---|---|
| With Fe3O4/SiO2/(3-chloropropyl)trimethoxysilane/2,2′-(4,4′-(propane-1,3-diyl)bis(piperazine-4,1-diyl))- diethanamine/Pd In acetonitrile for 13h; Catalytic behavior; Reflux; | A 91% B n/a |


| Conditions | Yield |
|---|---|
| With C8H14N2O4S In dichloromethane for 1h; Reflux; | 90% |
| With vanadium oxide on hydrotalcite (V/HT) In 1,3,5-trimethyl-benzene for 24h; Reflux; | 89% |
| Stage #1: 3-chlorobenzamide With C39H45N2 In acetonitrile at 20℃; Schlenk technique; Glovebox; Inert atmosphere; Stage #2: With phenylsilane In acetonitrile at 20℃; for 12h; Schlenk technique; Inert atmosphere; Sealed tube; | 82% |

| Conditions | Yield |
|---|---|
| With ammonium acetate; iodine at 100℃; for 3h; | 90% |
| With 2,3'-bipyridine; ammonium hydroxide; copper(l) iodide; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; oxygen In ethanol at 20℃; for 24h; | 85% |
| With potassium phosphate; ammonium formate In acetonitrile at 115℃; for 16h; Sealed tube; Green chemistry; | 82% |
| With copper(II) choride dihydrate; ammonium formate; potassium carbonate In neat (no solvent) at 135℃; under 760.051 Torr; for 24h; Sealed tube; Schlenk technique; Green chemistry; | 75% |

| Conditions | Yield |
|---|---|
| With [Pd{C6H4(CH2N(CH2Ph)2)}(μ-Br)]2; tetrabutylammomium bromide; potassium carbonate In N,N-dimethyl-formamide at 130℃; for 0.0833333h; Microwave irradiation; chemoselective reaction; | 90% |
| With C20H28Br2N2O4Pd2; potassium carbonate In N,N-dimethyl-formamide at 130℃; for 0.333333h; Inert atmosphere; Microwave irradiation; chemoselective reaction; | 58% |

| Conditions | Yield |
|---|---|
| With [Pd{C6H2(CH2CH2NH2)-(OMe)2-2,3}(μ-Br)]2; tetrabutylammomium bromide; potassium carbonate In N,N-dimethyl-formamide at 130℃; for 0.0833333h; Microwave irradiation; Green chemistry; chemoselective reaction; | 90% |
-
-
63503-60-6
3-chlorophenylboronic acid

-
-
119986-58-2
N-(4-chlorophenyl)-N-cyano-4-methylbenzenesulfonamide

-
A
-
766-84-7
3-chloro-benzonitrile

-
B
-
2903-34-6
4-methyl-N-(4-chlorophenyl)benzenesulfonamide

| Conditions | Yield |
|---|---|
| With Fe3O4/SiO2/(3-chloropropyl)trimethoxysilane/2,2′-(4,4′-(propane-1,3-diyl)bis(piperazine-4,1-diyl))- diethanamine/Pd In acetonitrile for 16h; Catalytic behavior; Reflux; | A 90% B n/a |

-
-
625-99-0
3-iodochlorobenzene

-
-
19555-13-6
2-(dimethylamino)malononitrile

-
-
766-84-7
3-chloro-benzonitrile

| Conditions | Yield |
|---|---|
| With copper(II) orthophosphate In N,N-dimethyl-formamide at 120℃; for 24h; Inert atmosphere; Sealed tube; | 89% |
-
-
587-04-2
3-Chlorobenzaldehyde

-
A
-
766-84-7
3-chloro-benzonitrile

-
B
-
34158-71-9
3-chlorobenzaldehyde oxime

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; FeOO280 In toluene for 7h; Condensation; dehydration; Heating; | A 86% B 14% |

| Conditions | Yield |
|---|---|
| With Bromotrichloromethane; trichloroisocyanuric acid; bromine at 10 - 120℃; for 0.5h; Photolysis; | A 84% B 10% |
-
-
4152-90-3
m-chlorobenzylamine

-
A
-
766-84-7
3-chloro-benzonitrile

-
B
-
161723-68-8
N-(3-chlorobenzylidene)-1-(3-chlorophenyl)methylamine

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; N,N-dimethylethylenediamine; copper(l) chloride In toluene at 80℃; for 24h; Sealed tube; Schlenk technique; chemoselective reaction; | A 82% B n/a |
| With iodine; tert-butylamine In acetonitrile for 6h; Ambient temperature; | A 86 % Chromat. B 9 % Chromat. |
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; C63H51N2O3P2Ru(1+)*ClO4(1-); oxygen In toluene at 90℃; under 760.051 Torr; for 20h; | A 50 %Chromat. B 20 %Chromat. |

| Conditions | Yield |
|---|---|
| With oxygen; copper(II) trifluoroacetate; urea In dimethyl sulfoxide at 110℃; for 38h; Green chemistry; | 80% |

| Conditions | Yield |
|---|---|
| With oxygen; copper(l) cyanide In dimethyl sulfoxide at 150℃; | 78% |

| Conditions | Yield |
|---|---|
| With aluminum oxide; aminosulfonic acid; urea for 0.0833333h; Irradiation; | 77% |
| With ammonium hydroxide; thionyl chloride 2.) dioxane, 3.) reflux; Multistep reaction; |
-
-
55305-43-6
N-cyano-N-phenyl-p-toluenesulfonamide

-
-
63503-60-6
3-chlorophenylboronic acid

-
-
766-84-7
3-chloro-benzonitrile

| Conditions | Yield |
|---|---|
| With hydroxo(1,5-cyclooctadiene)rhodium (I) dimer; potassium carbonate In 1,4-dioxane at 80℃; for 4h; Inert atmosphere; | 76% |

| Conditions | Yield |
|---|---|
| With [Ru(η6-C6Me6)Cl2(tris(dimethylamino)phosphine)]; hydroxylamine hydrochloride; sodium hydrogencarbonate In water at 100℃; for 7h; Inert atmosphere; Sealed tube; Green chemistry; | A 74% B n/a |

| Conditions | Yield |
|---|---|
| Stage #1: 1-bromo-3-chlorobenzene With TurboGrignard In tetrahydrofuran at 0℃; for 5h; Stage #2: 2,2-dimethylmalononitrile In tetrahydrofuran at 0 - 23℃; for 1h; | 73% |

| Conditions | Yield |
|---|---|
| With tetrachloromethane; bis(acetylacetonate)oxovanadium at 150℃; for 6h; Autoclave; | 72% |
-
-
3717-33-7, 34158-71-9, 4006-79-5
(E)-3-chlorobenzaldehyde oxime

-
-
75-05-8
acetonitrile

-
A
-
766-84-7
3-chloro-benzonitrile

-
B
-
95124-66-6
3-(3-chlorophenyl)-5-methyl-1,2,4-oxadiazole

-
C
-
535-80-8
3-chlorobenzoate

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate at 70℃; for 1h; | A n/a B 70% C 7% |

-
-
766-84-7
3-chloro-benzonitrile

-
-
22179-77-7
3-chloro-N-hydroxy-benzamidine

| Conditions | Yield |
|---|---|
| With hydroxylamine monohydrate In ethanol at 90℃; for 1h; Sealed tube; | 100% |
| With sodium hydroxide; hydroxylamine hydrochloride In ethanol; water at 80℃; for 2h; | 85.9% |
| With hydroxylamine hydrochloride; sodium hydrogencarbonate In ethanol; water at 25℃; for 70h; | 80% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 0 - 20℃; for 16.33h; | 100% |
| With hydrogenchloride at 0 - 20℃; for 16.3333h; | 100% |
| With hydrogenchloride at 0 - 20℃; for 16.3333h; | 100% |
-
-
766-84-7
3-chloro-benzonitrile

-
-
41421-28-7
5-(m-chlorophenyl)tetrazole

| Conditions | Yield |
|---|---|
| Stage #1: 3-chloro-benzonitrile With sodium azide In N,N-dimethyl-formamide at 120℃; for 36h; Stage #2: With hydrogenchloride In water; ethyl acetate for 0.0833333h; | 99% |
| With sodium azide at 120℃; for 0.0833333h; | 98% |
| With sodium azide at 130℃; for 6.75h; Catalytic behavior; | 98% |

| Conditions | Yield |
|---|---|
| With water; potassium carbonate at 150℃; for 0.25h; Microwave irradiation; | 99% |
| With C12H24O16Ru3*2H2O In water at 110℃; for 1h; Catalytic behavior; Schlenk technique; Inert atmosphere; Autoclave; | 99% |
| With polystyrene-triethylenetetramine anchored ruthenium(II) complex; air In water at 90℃; for 7h; Green chemistry; | 95% |
-
-
766-84-7
3-chloro-benzonitrile

-
-
42365-42-4
3-chloro-benzylamine; hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: 3-chloro-benzonitrile With [2,6-η6:η1-bis(2,4,6-trimethylphenyl)phenylthiolato]triethylphosphineruthenium(II)tetrakis[3,5-bis(trifluoromethyl)phenyl]borate; diethylphenylsilane at 20℃; for 18h; Glovebox; Inert atmosphere; Stage #2: With hydrogenchloride In diethyl ether at 20℃; for 1h; Glovebox; Inert atmosphere; | 99% |
| Stage #1: 3-chloro-benzonitrile With MnBr(CO)2[NH(CH2CH2P(iPr)2)2]; hydrogen; sodium t-butanolate In toluene at 120℃; under 37503.8 Torr; for 24h; Autoclave; Stage #2: With hydrogenchloride In diethyl ether | 96% |
| Stage #1: 3-chloro-benzonitrile With cobalt pivalate; 1,1,3,3-Tetramethyldisiloxane; tert-butylisonitrile at 80℃; for 24h; Stage #2: With hydrogenchloride In diethyl ether at 20℃; for 0.5h; | 95% |

| Conditions | Yield |
|---|---|
| With C16H36N(1+)*C32H25Cl2Fe2N2O3PdS(1-); potassium carbonate In water for 0.25h; Suzuki coupling; Microwave irradiation; | 99% |
| With caesium carbonate; tris(dibenzylideneacetone)dipalladium (0) In 1,4-dioxane for 1h; Suzuki-Miyaura coupling; Heating; | 98% |
| With potassium phosphate; 2-(diphenylphosphino)phenylferrocene; tris-(dibenzylideneacetone)dipalladium(0) In toluene at 90℃; for 24h; Suzuki-Miyaura coupling; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-chloro-benzonitrile; n-pentylmagnesium bromide In tetrahydrofuran; diethyl ether at 0 - 20℃; Inert atmosphere; Stage #2: With hydrogenchloride; water In tetrahydrofuran; diethyl ether at 0 - 20℃; | 99% |
-
-
766-84-7
3-chloro-benzonitrile

-
-
58521-27-0
pentafluorophenyl potassium carboxylate

| Conditions | Yield |
|---|---|
| With palladium diacetate; tricyclohexylphosphine In diethylene glycol dimethyl ether at 130℃; for 24h; Inert atmosphere; Sealed tube; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-chloro-benzonitrile; pentylmagnesium chloride In tetrahydrofuran; diethyl ether at 20℃; for 144h; Inert atmosphere; Stage #2: With hydrogenchloride; water In tetrahydrofuran; diethyl ether at 0℃; | 99% |

| Conditions | Yield |
|---|---|
| With Pd-PEPPSI-IPrAn In tetrahydrofuran; 1,4-dioxane at 0 - 20℃; for 0.5h; Negishi Coupling; Schlenk technique; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With tetrakis[μ-1-[(3-methoxyphenyl)methyl]-2-phenyl-3-[2-oxo-2-[(2-phenolato-kO)aminokN]ethyl]-1H-imidazoliumato-kC4]tetrapalladium; tetrabutylammomium bromide; sodium acetate at 140℃; for 16h; Heck Reaction; | 99% |
| With tetrabutylammomium bromide; sodium acetate; C84H60N12O8Pd4 at 140℃; for 2h; Heck Reaction; Inert atmosphere; Schlenk technique; | 99% |
-
-
35676-30-3
4,5,6,7-Tetrahydrocyclohexa-1,2,3-selenadiazol

-
-
766-84-7
3-chloro-benzonitrile

| Conditions | Yield |
|---|---|
| With chloro(1,5-cyclooctadiene)rhodium(I) dimer; (S,S)-2,3-O-isopropylidene-2,3-dihydroxy-1,4-bis(diphenylphosphino)butane In 1,4-dioxane at 100℃; for 6h; | 99% |
| With chloro(1,5-cyclooctadiene)rhodium(I) dimer; (S,S)-2,3-O-isopropylidene-2,3-dihydroxy-1,4-bis(diphenylphosphino)butane In 1,4-dioxane at 100℃; for 6h; | 99% |
| With chloro(1,5-cyclooctadiene)rhodium(I) dimer; (S,S)-2,3-O-isopropylidene-2,3-dihydroxy-1,4-bis(diphenylphosphino)butane In 1,4-dioxane at 100℃; for 6h; | 99% |

| Conditions | Yield |
|---|---|
| With 2-(2,6-dimethoxyphenyl)-1-methyl-3-(diphenylphosphino)-1H-indole; palladium diacetate; lithium tert-butoxide In 1,4-dioxane at 100℃; for 1h; Schlenk technique; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-chloro-benzonitrile; benzaldehyde With hydrogen In 2-methyltetrahydrofuran at 100℃; under 11251.1 Torr; for 20h; High pressure; Autoclave; Stage #2: With hydrogenchloride In diethyl ether | 99% |
-
-
766-84-7
3-chloro-benzonitrile

-
-
107-15-3
ethylenediamine

-
-
27429-86-3
2-(3-chlorophenyl)-4,5-dihydro-1H-imidazole

| Conditions | Yield |
|---|---|
| With tetraphosphorus decasulfide for 0.0416667h; microwave irradiation; | 98% |
| With toluene-4-sulfonic acid for 0.416667h; Reflux; neat (no solvent); | 92% |
| With sulfur at 20℃; for 0.116667h; ultrasonic irradiation; | 89% |
-
-
766-84-7
3-chloro-benzonitrile

-
-
156-87-6
propan-1-ol-3-amine

-
-
91552-24-8
2-(3-chlorophenyl)-5,6-dihydro-4H-1,3-oxazine

| Conditions | Yield |
|---|---|
| With Montmorillonite K-10 for 7h; Microwave irradiation; | 98% |
| With montmorillonite KSF at 30℃; for 0.166667h; Sonication; | 98% |
| With phosphotungstic acid for 0.121667h; Microwave irradiation; chemoselective reaction; | 90% |
-
-
766-84-7
3-chloro-benzonitrile

-
-
66117-64-4
di(p-tolyl)borinic acid

-
-
133909-96-3
4'-methyl-[1,1'-biphenyl]-3-carbonitrile

| Conditions | Yield |
|---|---|
| With NiCl2(Tris-(4-methoxy-phenyl)-phosphin)2; potassium phosphate tribasic trihydrate; P(p-CH3OC6H4)3 In toluene at 110℃; for 5h; Inert atmosphere; | 98% |
| With bis(triphenylphosphine)nickel(II) chloride; potassium phosphate tribasic trihydrate; 1-n-butyl-3-methylimidazolim bromide In toluene at 110℃; for 6h; Inert atmosphere; | 93% |
-
-
60474-27-3
ethyl 5-phenyl-1,2,3-thiadiazole-4-carboxylate

-
-
766-84-7
3-chloro-benzonitrile

| Conditions | Yield |
|---|---|
| With 1,1'-bis-(diphenylphosphino)ferrocene; chloro(1,5-cyclooctadiene)rhodium(I) dimer In chlorobenzene at 130℃; for 1h; Glovebox; | 98% |

| Conditions | Yield |
|---|---|
| With benzene-1,2-dicarboxylic acid for 0.666667h; microwave-irradiation; | 97% |
| With potassium hydroxide In ethylene glycol at 170℃; for 7h; |
-
-
766-84-7
3-chloro-benzonitrile

-
-
16419-60-6
2-Methylphenylboronic acid

-
-
253678-80-7
2'-methyl-[1,1'-biphenyl]-3-carbonitrile

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); potassium phosphate monohydrate; 2-(2-methoxyphenyl)-1-methyl-3-(diphenylphosphino)-1H-indole In 1,4-dioxane at 90℃; for 24h; Suzuki-Miyaura coupling; Inert atmosphere; | 97% |
| With (2’,4’,6’-triisopropyl-[1,1’-biphenyl]-2-yl)-diphenylphosphine; potassium phosphate; palladium diacetate In tetrahydrofuran at 20℃; for 24h; Suzuki-Miyaura coupling; | 94% |
| With potassium phosphate monohydrate; 1-methyl-2-(2-(dicyclohexylphosphino)phenyl)-1H-benzoimidazole; palladium diacetate In 1,4-dioxane; 1,3,5-trimethyl-benzene at 135℃; for 24h; Suzuki-Miyaura coupling; Inert atmosphere; | 81% |
-
-
766-84-7
3-chloro-benzonitrile

-
-
2234-82-4
1-propylmagnesium chloride

-
-
21550-08-3
1-(3-chlorophenyl)butan-1-one

| Conditions | Yield |
|---|---|
| Stage #1: 3-chloro-benzonitrile; 1-propylmagnesium chloride; copper(l) chloride In tetrahydrofuran; diethyl ether for 0.5h; Heating / reflux; Stage #2: With hydrogenchloride In tetrahydrofuran; diethyl ether; water at 20℃; Stage #3: With water; hydrogenchloride at 90℃; for 1h; | 97% |
| Stage #1: 3-chloro-benzonitrile; 1-propylmagnesium chloride In tetrahydrofuran; diethyl ether at 0 - 20℃; Inert atmosphere; Stage #2: With hydrogenchloride; water In tetrahydrofuran; diethyl ether at 0 - 20℃; | 88% |
| Stage #1: 3-chloro-benzonitrile; 1-propylmagnesium chloride In tetrahydrofuran; diethyl ether at 0 - 20℃; for 96h; Inert atmosphere; Stage #2: With hydrogenchloride; water In tetrahydrofuran; diethyl ether at 20℃; for 1h; | 88% |
-
-
766-84-7
3-chloro-benzonitrile

-
-
931-51-1
cyclohexylmagnesiumchloride

-
-
211985-77-2
(3-chlorophenyl)(cyclohexyl)methanone

| Conditions | Yield |
|---|---|
| Stage #1: 3-chloro-benzonitrile; cyclohexylmagnesiumchloride; copper(l) chloride In tetrahydrofuran; diethyl ether for 0.5h; Heating / reflux; Stage #2: With hydrogenchloride In tetrahydrofuran; diethyl ether; water at 20℃; Stage #3: With water; hydrogenchloride at 90℃; for 1h; | 97% |

| Conditions | Yield |
|---|---|
| With 1,3-bis(dicyclohexylphosphino)propane bis(tetrafluoroborate) salt; water; palladium diacetate; potassium carbonate In dimethyl sulfoxide at 100℃; under 760.051 Torr; for 15h; | 97% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-(2′,4′,6′-triisopropyl-3,6-dimethoxy-[1,1′-biphenyl]-2-yl)phosphine; boric acid; palladium diacetate; caesium carbonate In 1-methyl-pyrrolidin-2-one at 80℃; for 24h; Schlenk technique; Inert atmosphere; | 97% |
| With tris(6,6'-diamino-2,2'-bipyridine); 4,4-diphenyl-1,3,5,7,8-pentamethyl-2,6-diethyl-4-bora-3a,4a-diaza-s-indacene; Br2Ni*3H2O; water; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide; acetonitrile at 20℃; for 24h; Glovebox; Irradiation; Inert atmosphere; | 91% |
| With trans-di(μ-acetato)bis[o-(di-o-tolyl-phosphino)benzyl]dipalladium(II); C29H45Pt; potassium carbonate In water; N,N-dimethyl-formamide at 115℃; for 0.5h; Inert atmosphere; Microwave irradiation; | 90% |

| Conditions | Yield |
|---|---|
| With (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; 2.9-dimethyl-1,10-phenanthroline In 1,4-dioxane at 100℃; for 20h; Catalytic behavior; Reagent/catalyst; Inert atmosphere; Sealed tube; regioselective reaction; | 97% |
-
-
766-84-7
3-chloro-benzonitrile

-
-
22179-77-7
3-chloro-N-hydroxybenzimidamide

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium carbonate In ethanol; water for 6h; Reflux; | 96% |
| With hydroxylamine hydrochloride; potassium carbonate In ethanol | |
| With hydroxylamine hydrochloride; sodium carbonate In ethanol Heating; |
-
-
6773-29-1
dimethyl diazomalonate

-
-
766-84-7
3-chloro-benzonitrile

-
-
109345-74-6
4-Carbomethoxy-5-methoxy-2-(3'-chlorophenyl)oxazole

| Conditions | Yield |
|---|---|
| dirhodium tetraacetate In chloroform for 8h; Heating; | 96% |
| dirhodium tetraacetate In chloroform Heating; | 96% |

| Conditions | Yield |
|---|---|
| With sodium monohydrogen sulfide x-hydrate; magnesium(II) chloride hexahydrate In N,N-dimethyl-formamide at 20℃; for 2h; | 96% |
| With diammonium sulfide; 1,6-bis(3-methylimidazolium-1-yl)hexane dichloride at 70℃; for 0.05h; | 94% |
| With diphosphorus pentasulfide; ethanol for 4h; Heating; | 88% |
3-Chlorobenzonitrile Specification
The 3-Chlorobenzonitrile with CAS registry number of 766-84-7 is also known as Benzonitrile, m-chloro-. The IUPAC name and product name are the same. It belongs to product categories of Boron, Nitrile, Thio,& TM-Cpds; Halides; Aromatic Nitriles; Chlorine Compounds; Nitriles; C6 to C7; Cyanides/Nitriles; Nitrogen Compounds. Its EINECS registry number is 212-172-6. In addition, the formula is C7H4ClN and the molecular weight is 137.57. This chemical is a white crystal and should be sealed in a ventilated and dry place.
Physical properties about 3-Chlorobenzonitrile are: (1)ACD/LogP: 2.35; (2)ACD/LogD (pH 5.5): 2.35; (3)ACD/LogD (pH 7.4): 2.35; (4)ACD/BCF (pH 5.5): 35.98; (5)ACD/BCF (pH 7.4): 35.98; (6)ACD/KOC (pH 5.5): 452.29; (7)ACD/KOC (pH 7.4): 452.29; (8)#H bond acceptors: 1; (9)Index of Refraction: 1.563; (10)Molar Refractivity: 36.14 cm3; (11)Molar Volume: 111.2 cm3; (12)Surface Tension: 46.2 dyne/cm; (13)Density: 1.23 g/cm3; (14)Flash Point: 97.2 °C; (15)Enthalpy of Vaporization: 43.97 kJ/mol; (16)Boiling Point: 203.4 °C at 760 mmHg; (17)Vapour Pressure: 0.278 mmHg at 25 °C.
Preparation of 3-Chlorobenzonitrile: it is prepared by reaction of 3-chloro-benzamide. The reaction needs reagent 2-(trifluoroacetyloxy)pyridine and solventacetonitrile and other condition of heating for 5 hours. The yield is about 77%.

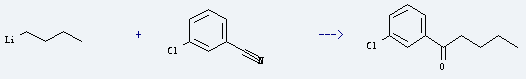
Uses of 3-Chlorobenzonitrile: it is used as intermediate of medicine, pesticide and dye. It is used to produce 1-(m-chlorophenyl)pentan-1-one by reaction with butyllithium. The reaction occurs with reagent hexane and diethyl ether at ambient temperature. The yield is about 67%.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes and harmful in contact with skin and if swallowed. During using it, do not breathe gas/fumes/vapour/spray and avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=CC(=CC(=C1)Cl)C#N
2. InChI: InChI=1S/C7H4ClN/c8-7-3-1-2-6(4-7)5-9/h1-4H
3. InChIKey: WBUOVKBZJOIOAE-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 800mg/kg (800mg/kg) | Farmaco, Edizione Scientifica. Vol. 41, Pg. 41, 1986. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View