-
Name
3-Furaldehyde
- EINECS
- CAS No. 498-60-2
- Article Data41
- CAS DataBase
- Density 1.145 g/cm3
- Solubility Soluble in water, benzene, chloroform, alcohol and ether.
- Melting Point 148-149.5 °C
- Formula C5H4O2
- Boiling Point 145.3 °C at 760 mmHg
- Molecular Weight 96.0856
- Flash Point 48.3 °C
- Transport Information UN 1989 3/PG 3
- Appearance Colorless to light yellow liquid
- Safety 26-36-45-36/37-16-3
- Risk Codes 10-36/37/38-23/25-21
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi,  F,
F,  T
T
- Synonyms 3-Furylcarboxaldehyde;3-Furylaldehyde;3-Furfural;3-Formylfuran;3-Furaldehyde(7CI,8CI);Furan-3-carbaldehyde;
- PSA 30.21000
- LogP 1.09210
Synthetic route
-
-
859077-01-3
3-Diacetoxymethyl-furan

-
-
498-60-2
furan-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| With iron(II) sulfate In benzene for 0.25h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| With 4,4'-bis-(dichloroiodo)-biphenyl; tetraethylammonium bromide In chloroform at 20℃; for 0.75h; | 90% |
| With calcium persulfate; silica gel for 0.0833333h; microwave irradiation; | 90% |
| With silica gel; 4-acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium perchlorate In dichloromethane Ambient temperature; | 89% |

| Conditions | Yield |
|---|---|
| In methanol decomposition of the complex by refluxing in methanol under N2 for 6-8 h; evapn. of the solvent, extn. of the organic compounds with ether, gas chromatography; | A 15% B 85% |
-
-
99595-62-7
Methyl 3-(3'-furanyl)prop-2-en-1-oate

-
-
498-60-2
furan-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| With oxygen; palladium diacetate; toluene-4-sulfonic acid In water at 100℃; under 6080.41 Torr; for 24h; Autoclave; | 69% |
-
-
26214-65-3
furan-3-carbonyl chloride

-
-
498-60-2
furan-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| With tetrahydrogenoboratebis(triphenylphosphine)copper(I); triphenylphosphine In acetone for 1h; Ambient temperature; | 67% |
| With Pd-BaSO4; thiourea; xylene Hydrogenation; |

| Conditions | Yield |
|---|---|
| With rhodium(III) chloride trihydrate; hydrogen; triethylamine; triphenylphosphine In N,N-dimethyl acetamide at 90℃; under 7500.75 Torr; for 12h; Autoclave; | 66% |
| With tri-n-butyl-tin hydride; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran at 50℃; under 2280 Torr; | 60% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-bromofurane With n-butyllithium In 2-methyltetrahydrofuran; dodecane; hexane at -75℃; for 0.333333h; Inert atmosphere; Stage #2: N,N-dimethyl-formamide In 2-methyltetrahydrofuran; dodecane; hexane at -75 - 20℃; Heating; | 65% |

| Conditions | Yield |
|---|---|
| With Bis(N-methylpiperazinyl) aluminum hydride In tetrahydrofuran for 8h; Heating; | 62% |
| With hydrogen; 2,2-dimethylpropanoic anhydride; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran at 80℃; under 22501.8 Torr; for 48h; | 90 % Spectr. |
| Multi-step reaction with 2 steps 1: 83 percent / LiAlH4 / tetrahydrofuran / 22 h / Heating 2: 80 percent / CrO3, pyridine / CH2Cl2 / 0.33 h View Scheme |

| Conditions | Yield |
|---|---|
| With rhodium(III) iodide; hydrogen; acetic anhydride; triethylamine; triphenylphosphine In N,N-dimethyl acetamide at 100℃; for 24h; Autoclave; | 38% |

| Conditions | Yield |
|---|---|
| With rhodium(III) iodide; dichloro [1,1'-bis(diphenylphosphino)propane]palladium(II); hydrogen; acetic anhydride; triethylamine; triphenylphosphine In N,N-dimethyl acetamide at 100℃; for 24h; Autoclave; | 35% |
-
-
50-99-7, 59-23-4, 921-60-8, 1949-88-8, 1990-29-0, 2152-76-3, 2595-97-3, 2595-98-4, 3458-28-4, 4205-23-6, 5934-56-5, 5978-95-0, 5987-68-8, 6027-89-0, 6038-51-3, 7635-11-2, 10030-80-5, 15572-79-9, 19163-87-2, 23567-25-1, 26566-61-0, 30077-17-9, 31103-86-3, 39665-52-6, 40866-07-7, 58367-01-4, 58407-05-9, 58407-06-0, 83198-69-0, 83198-70-3, 83198-71-4, 93780-23-5, 145920-48-5
glucose

-
A
-
498-60-2
furan-3-carboxaldehyde

-
B
-
116-09-6
hydroxy-2-propanone

| Conditions | Yield |
|---|---|
| With hydrogen; copper chromite In water at 240 - 280℃; under 750.075 Torr; Product distribution / selectivity; Gas phase; | A 5.7% B 2.5% |
-
-
104322-42-1
(-)-(2S)-benzyloxy-2,5-dihydrofuran-4-carboxaldehyde

-
-
498-60-2
furan-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| With Duolit reasin In dichloromethane for 1h; |
-
-
104322-41-0
(+)-(2R)-benzyloxy-2,5-dihydrofuran-4-carboxaldehyde

-
-
498-60-2
furan-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| With Duolit resin In dichloromethane for 1h; |
-
-
81311-92-4
3-Furan-3-yl-2,3-dihydroxy-N-(2-methoxy-5-oxo-cyclopent-1-enyl)-N-methyl-propionamide

-
-
498-60-2
furan-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| With sodium periodate In ethanol; water at 17℃; for 1h; |
-
-
859077-01-3
3-Diacetoxymethyl-furan

-
-
498-60-2
furan-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| With iron(II) sulfate In benzene for 0.25h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| With 4,4'-bis-(dichloroiodo)-biphenyl; tetraethylammonium bromide In chloroform at 20℃; for 0.75h; | 90% |
| With calcium persulfate; silica gel for 0.0833333h; microwave irradiation; | 90% |
| With silica gel; 4-acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium perchlorate In dichloromethane Ambient temperature; | 89% |

| Conditions | Yield |
|---|---|
| In methanol decomposition of the complex by refluxing in methanol under N2 for 6-8 h; evapn. of the solvent, extn. of the organic compounds with ether, gas chromatography; | A 15% B 85% |
-
-
99595-62-7
Methyl 3-(3'-furanyl)prop-2-en-1-oate

-
-
498-60-2
furan-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| With oxygen; palladium diacetate; toluene-4-sulfonic acid In water at 100℃; under 6080.41 Torr; for 24h; Autoclave; | 69% |
-
-
26214-65-3
furan-3-carbonyl chloride

-
-
498-60-2
furan-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| With tetrahydrogenoboratebis(triphenylphosphine)copper(I); triphenylphosphine In acetone for 1h; Ambient temperature; | 67% |
| With Pd-BaSO4; thiourea; xylene Hydrogenation; |

| Conditions | Yield |
|---|---|
| With rhodium(III) chloride trihydrate; hydrogen; triethylamine; triphenylphosphine In N,N-dimethyl acetamide at 90℃; under 7500.75 Torr; for 12h; Autoclave; | 66% |
| With tri-n-butyl-tin hydride; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran at 50℃; under 2280 Torr; | 60% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-bromofurane With n-butyllithium In 2-methyltetrahydrofuran; dodecane; hexane at -75℃; for 0.333333h; Inert atmosphere; Stage #2: N,N-dimethyl-formamide In 2-methyltetrahydrofuran; dodecane; hexane at -75 - 20℃; Heating; | 65% |

| Conditions | Yield |
|---|---|
| With Bis(N-methylpiperazinyl) aluminum hydride In tetrahydrofuran for 8h; Heating; | 62% |
| With hydrogen; 2,2-dimethylpropanoic anhydride; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran at 80℃; under 22501.8 Torr; for 48h; | 90 % Spectr. |
| Multi-step reaction with 2 steps 1: 83 percent / LiAlH4 / tetrahydrofuran / 22 h / Heating 2: 80 percent / CrO3, pyridine / CH2Cl2 / 0.33 h View Scheme |

| Conditions | Yield |
|---|---|
| With rhodium(III) iodide; hydrogen; acetic anhydride; triethylamine; triphenylphosphine In N,N-dimethyl acetamide at 100℃; for 24h; Autoclave; | 38% |

| Conditions | Yield |
|---|---|
| With rhodium(III) iodide; dichloro [1,1'-bis(diphenylphosphino)propane]palladium(II); hydrogen; acetic anhydride; triethylamine; triphenylphosphine In N,N-dimethyl acetamide at 100℃; for 24h; Autoclave; | 35% |
-
-
50-99-7, 59-23-4, 921-60-8, 1949-88-8, 1990-29-0, 2152-76-3, 2595-97-3, 2595-98-4, 3458-28-4, 4205-23-6, 5934-56-5, 5978-95-0, 5987-68-8, 6027-89-0, 6038-51-3, 7635-11-2, 10030-80-5, 15572-79-9, 19163-87-2, 23567-25-1, 26566-61-0, 30077-17-9, 31103-86-3, 39665-52-6, 40866-07-7, 58367-01-4, 58407-05-9, 58407-06-0, 83198-69-0, 83198-70-3, 83198-71-4, 93780-23-5, 145920-48-5
glucose

-
A
-
498-60-2
furan-3-carboxaldehyde

-
B
-
116-09-6
hydroxy-2-propanone

| Conditions | Yield |
|---|---|
| With hydrogen; copper chromite In water at 240 - 280℃; under 750.075 Torr; Product distribution / selectivity; Gas phase; | A 5.7% B 2.5% |
-
-
104322-42-1
(-)-(2S)-benzyloxy-2,5-dihydrofuran-4-carboxaldehyde

-
-
498-60-2
furan-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| With Duolit reasin In dichloromethane for 1h; |
-
-
104322-41-0
(+)-(2R)-benzyloxy-2,5-dihydrofuran-4-carboxaldehyde

-
-
498-60-2
furan-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| With Duolit resin In dichloromethane for 1h; |
-
-
81311-92-4
3-Furan-3-yl-2,3-dihydroxy-N-(2-methoxy-5-oxo-cyclopent-1-enyl)-N-methyl-propionamide

-
-
498-60-2
furan-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| With sodium periodate In ethanol; water at 17℃; for 1h; |

-
A
-
98-01-1
furfural

-
B
-
498-60-2
furan-3-carboxaldehyde

-
C
-
271-89-6
1-benzofurane

-
D
-
497-23-4
2-buten-4-olide

| Conditions | Yield |
|---|---|
| With air at 400 - 600℃; Oxidation; Formation of xenobiotics; |

-
-
65359-87-7
benzyl 2,3-anhydro-β-L-ribopyranoside

-
-
498-60-2
furan-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N,N,N',N'-tetramethylurea, lithium bromide / toluene / 0.13 h / Heating 2: Duolit reasin / CH2Cl2 / 1 h View Scheme | |
| Multi-step reaction with 4 steps 1: 91 percent / dimethylsulfoxide, oxalyl chloride / CH2Cl2 / 0.25 h / -60 °C 2: 27 percent / sodium borodeuteride / methanol / 0.75 h 3: N,N,N',N'-tetramethylurea, lithium bromide / toluene / 0.13 h / Heating 4: Duolit reasin / CH2Cl2 / 1 h View Scheme |
-
-
79974-79-1
benzyl 2,3-anhydro-β-L-erythropentopyranosid-4-ulose

-
-
498-60-2
furan-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 27 percent / sodium borodeuteride / methanol / 0.75 h 2: N,N,N',N'-tetramethylurea, lithium bromide / toluene / 0.13 h / Heating 3: Duolit reasin / CH2Cl2 / 1 h View Scheme |
-
-
104292-65-1
benzyl 3,4-anhydro-β-L-ribopyranoside

-
-
498-60-2
furan-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 34 percent / N,N,N',N'-tetranethylurea, lithium bromide / toluene / 0.13 h / Heating 2: Duolit reasin / CH2Cl2 / 1 h View Scheme |
-
-
104292-63-9
benzyl 4-bromo-4-deoxy-α-D-lyxopyranoside

-
-
498-60-2
furan-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 90 percent / sodium methoxide / methanol / 1 h / Ambient temperature 2: 34 percent / N,N,N',N'-tetranethylurea, lithium bromide / toluene / 0.13 h / Heating 3: Duolit reasin / CH2Cl2 / 1 h View Scheme |

-
-
498-60-2
furan-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: N,N,N',N'-tetramethylurea, lithium bromide / toluene / 0.13 h / Heating 2: Duolit reasin / CH2Cl2 / 1 h View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: copper-powder; quinoline 2: benzene; thionyl chloride 3: palladium/barium sulfate; xylene; thiourea / Hydrogenation View Scheme |
-
-
187737-37-7
propene

-
A
-
98-01-1
furfural

-
B
-
498-60-2
furan-3-carboxaldehyde

-
C
-
79-41-4
poly(methacrylic acid)

-
D
-
999-55-3
allyl acrylate

-
E
-
100-52-7
benzaldehyde

-
F
-
64-19-7
acetic acid

-
G
-
802294-64-0
propionic acid

-
H
-
79-10-7
acrylic acid

-
I
-
107-02-8
acrolein

| Conditions | Yield |
|---|---|
| Gas phase; |

-
-
187737-37-7
propene

-
-
74-98-6
propane

-
-
75-19-4
cyclopropane

-
A
-
98-01-1
furfural

-
B
-
498-60-2
furan-3-carboxaldehyde

-
C
-
79-41-4
poly(methacrylic acid)

-
D
-
999-55-3
allyl acrylate

-
E
-
100-52-7
benzaldehyde

-
F
-
64-19-7
acetic acid

-
G
-
802294-64-0
propionic acid

-
H
-
79-10-7
acrylic acid

-
I
-
107-02-8
acrolein

| Conditions | Yield |
|---|---|
| With water; oxygen; [Bi2W2O9. 2WO3]0.5 [Mo12Co5.5Fe2.94Si1.59K0.08Ox]1, Mo12V3W1.2Cu2.4,4Ox Conversion of starting material; |


-
-
498-60-2
furan-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| In benzene decomposition of the complex by refluxing in benzene under N2 for 6-8 h; evapn. of the solvent, extn. of the organic compound with ether, gas chromatography; |

-
-
498-60-2
furan-3-carboxaldehyde

| Conditions | Yield |
|---|---|
| With Na0274MnO2*6H2O; oxygen In butan-1-ol at 100℃; under 760.051 Torr; for 4h; Green chemistry; | 94 %Chromat. |

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-pentenoate; acetaldehyde With iron(III) chloride; palladium on activated charcoal; acetic acid In methanol at 40 - 90℃; for 8h; Stage #2: With diisobutylaluminium hydride |

| Conditions | Yield |
|---|---|
| Stage #1: methyl methacrylate; acetaldehyde With palladium diacetate; acetic acid; copper dichloride In isopropyl alcohol at 40 - 90℃; for 8.5h; Stage #2: With sodium bis(2-methoxyethoxy)aluminium dihydride Reagent/catalyst; Solvent; |

| Conditions | Yield |
|---|---|
| Stage #1: acetaldehyde; acrylic acid methyl ester With hydrogenchloride; ruthenium trichloride; palladium on activated charcoal In methanol at 40 - 90℃; for 4h; Stage #2: With diisobutylaluminium hydride |
-
-
498-60-2
furan-3-carboxaldehyde

-
-
1099-45-2
ethyl (triphenylphosphoranylidene)acetate

-
-
58963-70-5
(E)-3-Furan-3-yl-acrylic acid ethyl ester

| Conditions | Yield |
|---|---|
| In benzene Heating; | 100% |
| In tetrahydrofuran for 2h; Wittig reaction; Reflux; | 99% |
| In dichloromethane at 20℃; Wittig Olefination; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at -78℃; | 100% |


| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol under 2585.74 Torr; for 24h; | 100% |

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol under 2585.74 Torr; for 24h; | 100% |
-
-
498-60-2
furan-3-carboxaldehyde

-
-
5927-18-4
trimethyl phosphonoacetate

-
-
99595-62-7
Methyl 3-(3'-furanyl)prop-2-en-1-oate

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 0℃; | 100% |

| Conditions | Yield |
|---|---|
| With acetic acid; N-butylamine for 0.5h; Henry Nitro Aldol Condensation; Molecular sieve; Reflux; | 100% |
| With ammonium acetate at 90℃; for 1h; Henry reaction; Microwave irradiation; | 83% |
| With sodium methylate at 0℃; for 0.0833333h; |

| Conditions | Yield |
|---|---|
| Stage #1: trimethylsulfoxonium iodide With potassium hydroxide; water In acetonitrile at 40℃; for 0.5h; Stage #2: furan-3-carboxaldehyde In tetrahydrofuran at 40℃; for 18h; | 100% |
-
-
498-60-2
furan-3-carboxaldehyde

-
-
1250851-46-7
(S)-(tert-butyl) 2-(2-oxopropyl)pyrrolidine-1-carboxylate

-
-
1621089-87-9
(2S)-(tert-butyl) 2-[4-(furan-3-yl)-4-hydroxy-2-oxobutyl]pyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl (2S)-2-(2'-oxopropyl)pyrrolidine-1-carboxylate With di-n-butylboryl trifluoromethanesulfonate; N-ethyl-N,N-diisopropylamine In diethyl ether at -78℃; for 1h; Inert atmosphere; Stage #2: furan-3-carboxaldehyde In diethyl ether at -78℃; for 5h; Inert atmosphere; Stage #3: With dihydrogen peroxide In methanol; aq. phosphate buffer at 0 - 20℃; for 1h; pH=7.2; Inert atmosphere; | 100% |
-
-
498-60-2
furan-3-carboxaldehyde

-
-
18295-60-8
allenylmagnesium bromide

-
-
196957-16-1
3-(1-hydroxybut-3-ynyl)furan

| Conditions | Yield |
|---|---|
| With mercury dichloride at -78 - 0℃; | 99% |
| In diethyl ether for 1h; Ambient temperature; | 98% |
| at -78 - 0℃; |
-
-
498-60-2
furan-3-carboxaldehyde

-
-
1099-45-2
ethyl (triphenylphosphoranylidene)acetate

-
-
58963-70-5
trans-3-furan-3-yl-acrylic acid ethyl ester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 2h; Heating / reflux; | 99% |
| In dichloromethane for 2h; | 96% |
| In dichloromethane | 94% |
-
-
498-60-2
furan-3-carboxaldehyde

-
-
106-95-6
allyl bromide

-
-
283612-33-9
(+/-)-1-(furan-3-yl)but-3-en-1-ol

| Conditions | Yield |
|---|---|
| With ammonium chloride; zinc In tetrahydrofuran Barbier reaction; | 99% |
| With ammonium chloride; zinc In tetrahydrofuran; water at 20℃; for 1h; | 98% |
| With ammonium chloride; zinc In tetrahydrofuran; water at 25℃; Barbier reaction; | 95% |
| With indium; sodium iodide In N,N-dimethyl-formamide at 20℃; | |
| With zinc In tetrahydrofuran at 0 - 20℃; |

| Conditions | Yield |
|---|---|
| Stage #1: propargyl bromide With magnesium; mercury dichloride In diethyl ether at 0℃; for 3h; Stage #2: furan-3-carboxaldehyde In diethyl ether at -78 - 0℃; | 99% |
| Stage #1: propargyl bromide With magnesium; mercury dichloride In diethyl ether; toluene at 0℃; for 4h; Inert atmosphere; Stage #2: furan-3-carboxaldehyde In diethyl ether; toluene at -78 - 0℃; for 0.666667h; Inert atmosphere; | 97% |
| With zinc In diethyl ether; N,N-dimethyl-formamide; toluene at 20℃; for 12h; Inert atmosphere; | 94% |
-
-
498-60-2
furan-3-carboxaldehyde

-
-
4301-14-8
acetylenemagnesium bromide

-
-
169377-39-3
1-(furan-3-yl)-1-hydroxy-2-propyne

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 20℃; for 5h; | 99% |
| In tetrahydrofuran at 0 - 23℃; Grignard reaction; | 99% |
| With ammonium chloride In tetrahydrofuran | |
| In tetrahydrofuran at 0 - 25℃; | |
| With ammonium chloride In tetrahydrofuran |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; water In diethyl ether at 0℃; for 0.5h; | 99% |
| With sodium tetrahydroborate; water In diethyl ether at 0 - 20℃; for 0.5h; | 99% |
| With formic acid; [Ir(2,2':6',2'’-terpyridine)(1,10-phenanthroline)Cl](PF6)2; sodium formate In ethanol; water at 100℃; for 0.25h; pH=Ca. 5; Microwave irradiation; chemoselective reaction; | 98% |
-
-
498-60-2
furan-3-carboxaldehyde

-
-
71699-33-7
5-hydroxy-4,6-dimethyl-3-heptanone

| Conditions | Yield |
|---|---|
| Stage #1: furan-3-carboxaldehyde With samarium diiodide In tetrahydrofuran at 20℃; for 0.5h; Inert atmosphere; Stage #2: 5-hydroxy-4,6-dimethyl-3-heptanone In tetrahydrofuran at -15℃; for 1h; Evans-Tishchenko coupling reaction; Inert atmosphere; optical yield given as %de; diastereoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With (2S,5R)-2-(methylaminomethyl)-1-methyl-5-phenylpyrrolidine; triethylamine; copper(ll) bromide In tetrahydrofuran at -25℃; for 72h; Henry Nitro Aldol Condensation; Inert atmosphere; Schlenk technique; enantioselective reaction; | 99% |
-
-
498-60-2
furan-3-carboxaldehyde

-
-
35761-91-2
5-iodo-1-trimethylsilyl-1-pentyne

| Conditions | Yield |
|---|---|
| Stage #1: 5-iodo-1-trimethylsilyl-1-pentyne With tert.-butyl lithium In diethyl ether; pentane at -78℃; for 1h; Inert atmosphere; Stage #2: furan-3-carboxaldehyde In diethyl ether; pentane at -78 - 20℃; for 1h; Inert atmosphere; | 99% |
-
-
498-60-2
furan-3-carboxaldehyde

-
-
925-90-6
ethylmagnesium bromide

-
-
66346-65-4
1-(furan-3-yl)propan-1-ol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether at 0℃; for 3h; | 98% |
| In tetrahydrofuran; diethyl ether at -20℃; for 2h; |
3-Furaldehyde Specification
The 3-Furaldehyde with CAS registry number of 498-60-2 is also known as 3-Furylcarboxaldehyde. The IUPAC name is Furan-3-carbaldehyde. It belongs to product categories of Aromatic Aldehydes & Derivatives (substituted); Furan&Benzofuran; API intermediates. In addition, the formula is C5H4O2 and the molecular weight is 96.08. This chemical is a colorless to light yellow liquid and should be sealed in ventilated and dry place away from oxides at the temperature of 2-8 °C.
Physical properties about 3-Furaldehyde are: (1)ACD/LogP: 0.51; (2)ACD/LogD (pH 5.5): 0.51; (3)ACD/LogD (pH 7.4): 0.51; (4)ACD/BCF (pH 5.5): 1.44; (5)ACD/BCF (pH 7.4): 1.44; (6)ACD/KOC (pH 5.5): 45.19; (7)ACD/KOC (pH 7.4): 45.19; (8)#H bond acceptors: 2; (9)#Freely Rotating Bonds: 1; (10)Index of Refraction: 1.515; (11)Molar Refractivity: 25.3 cm3; (12)Molar Volume: 83.8 cm3; (13)Surface Tension: 36.5 dyne/cm; (14)Density: 1.145 g/cm3; (15)Flash Point: 48.3 °C; (16)Enthalpy of Vaporization: 38.24 kJ/mol; (17)Boiling Point: 145.3 °C at 760 mmHg; (18)Vapour Pressure: 4.88 mmHg at 25 °C.
Preparation of 3-Furaldehyde: it is prepared by reaction of 3-iodo-furan with carbon monoxide. The reaction needs reagent Bu3SnH and solvent tetrahydrofuran over a Pd(PPh3)4 catalyst at the temperature of 50 °C. The yield is about 60%.
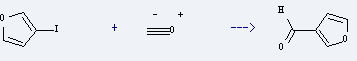
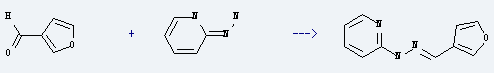
Uses of 3-Furaldehyde: it is used to produce N-furan-3-ylmethylene-N'-pyridin-2-yl-hydrazine by reaction with 2-hydrazino-pyridine. The reaction occurs with solvent ethanol and other condition of heating on a steam bath for 30 minutes. The yield is about 83%.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. What's more, it is toxic by inhalation and if swallowed. It is also flammable. During using it, wear suitable protective clothing and gloves. Keep in a cool place away from sources of ignition. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. If accident happens or you feel unwell seek medical advice immediately.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=COC=C1C=O
2. InChI: InChI=1S/C5H4O2/c6-3-5-1-2-7-4-5/h1-4H
3. InChIKey: AZVSIHIBYRHSLB-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View