-
Name
3-Methoxysalicylaldehyde
- EINECS 205-715-3
- CAS No. 148-53-8
- Article Data50
- CAS DataBase
- Density 1.231 g/cm3
- Solubility slightly soluble in water
- Melting Point 40-42 °C(lit.)
- Formula C8H8O3
- Boiling Point 265.5 °C at 760 mmHg
- Molecular Weight 152.15
- Flash Point 94 °C
- Transport Information
- Appearance Pale yellow to brown low melting solid
- Safety 23-36-24/25-36/37/39-27-26
- Risk Codes 22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms m-Anisaldehyde,2-hydroxy- (8CI);o-Vanillin (6CI);2-Hydroxy-3-methoxybenzaldehyde;2-Hydroxy-m-anisaldehyde;2-Vanillin;3-Methoxy-2-hydroxybenzaldehyde;6-Formyl-2-methoxyphenol;6-Formylguaiacol;NC 005;NSC 2150;Benzaldehyde,2-hydroxy-3-methoxy-;
- PSA 46.53000
- LogP 1.21330
Synthetic route

| Conditions | Yield |
|---|---|
| With lithium chloride In N,N-dimethyl-formamide for 22h; Heating; | 98% |
| With aluminum (III) chloride In dichloromethane at -5 - 25℃; | 94% |
| With cerium(III) chloride; sodium iodide In acetonitrile for 7h; dealkylation; Heating; | 89% |
| With magnesium iodide for 10h; neat (no solvent); | 80% |
| With n-butyllithium; lithium 4-methylpiperazin-1-ide In toluene 1.) 15 deg C, 20 min, 2.) 65-70 deg C; | 55% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 0.0333333h; Microwave irradiation; | 98% |
| With oxygen In aq. buffer at 45℃; for 13h; pH=4.5; Reagent/catalyst; Green chemistry; | 93 %Chromat. |
-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
217813-03-1
3-methoxy-2-(trimethylsilyl)phenyl-trifluoromethanesulfonate

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: N,N-dimethyl-formamide; 3-methoxy-2-(trimethylsilyl)phenyl-trifluoromethanesulfonate With tetrabutyl ammonium fluoride at 20℃; for 3h; Inert atmosphere; Stage #2: With water | 84% |

| Conditions | Yield |
|---|---|
| With iron(III) chloride In benzene Heating; | 77% |
-
-
2591-86-8
N-Formylpiperidine

-
-
217813-03-1
3-methoxy-2-(trimethylsilyl)phenyl-trifluoromethanesulfonate

-
A
-
32040-06-5
N-(3-methoxyphenyl)piperidine

-
B
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: N-Formylpiperidine; 3-methoxy-2-(trimethylsilyl)phenyl-trifluoromethanesulfonate With tetrabutyl ammonium fluoride In acetonitrile at 20℃; for 3h; Inert atmosphere; Stage #2: With water at 20℃; | A 6% B 60% |

-
-
24677-78-9
2,3-dihydroxybenzaldehyde

-
-
74-88-4
methyl iodide

-
A
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
B
-
86-51-1
2,3-dimethyoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydride In dimethyl sulfoxide at 25℃; for 17h; NaH : pyrocatechlol 2.2; Yields of byproduct given; | A 58% B n/a |

-
-
123-39-7
N-Methylformamide

-
-
217813-03-1
3-methoxy-2-(trimethylsilyl)phenyl-trifluoromethanesulfonate

-
A
-
150-19-6
O-methylresorcine

-
B
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: N-Methylformamide; 3-methoxy-2-(trimethylsilyl)phenyl-trifluoromethanesulfonate With tetrabutyl ammonium fluoride In acetonitrile at 20℃; for 3h; Inert atmosphere; Stage #2: With water at 20℃; | A 52% B 4% |


| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 20℃; for 12h; | 46% |
-
-
100-97-0
hexamethylenetetramine

-
-
90-05-1
2-methoxy-phenol

-
A
-
2931-90-0
5-formylvanillin

-
B
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
C
-
121-33-5
vanillin

| Conditions | Yield |
|---|---|
| Stage #1: hexamethylenetetramine; 2-methoxy-phenol With copper(I) oxide In trifluoroacetic acid for 5h; Duff Aldehyde Synthesis; Reflux; Stage #2: With hydrogenchloride In water at 20℃; for 1h; regioselective reaction; | A 9.5% B 11.5% C 38% |

-
-
5464-77-7
N,N-dibenzylformamide

-
-
217813-03-1
3-methoxy-2-(trimethylsilyl)phenyl-trifluoromethanesulfonate

-
A
-
56511-50-3
N-(3-methoxyphenyl)dibenzylamine

-
B
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: N,N-dibenzylformamide; 3-methoxy-2-(trimethylsilyl)phenyl-trifluoromethanesulfonate With tetrabutyl ammonium fluoride In acetonitrile at 20℃; for 3h; Inert atmosphere; Stage #2: With water at 20℃; | A 16% B 34% |

-
-
861779-85-3
3-methoxy-2-methoxycarbonyloxy-benzoyl chloride

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| With Pd-BaSO4; toluene at 110℃; Hydrogenation.und folgende Verseifung mit waessrig-alkoh.Natronlauge; |
-
-
54043-60-6
1,3,5-tris(o-tolyl)-hexahydro-s-triazine

-
-
90-05-1
2-methoxy-phenol

-
A
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
B
-
121-33-5
vanillin

| Conditions | Yield |
|---|---|
| und Erhitzen der Reaktionsprodukte mit Nitrobenzol und wss.NaOH auf 120-130grad; |

-
-
32752-36-6
1,3,5-tris-(4-ethoxy-phenyl)-hexahydro-[1,3,5]triazine

-
-
90-05-1
2-methoxy-phenol

-
A
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
B
-
121-33-5
vanillin

| Conditions | Yield |
|---|---|
| und Erhitzen der Reaktionsprodukte mit Nitrobenzol und wss.NaOH auf 120-130grad; |


| Conditions | Yield |
|---|---|
| With sodium hydroxide; water Behandeln des Reaktionsprodukts mit Aether, mit Natriumdisulfit und anschliessend mit H2SO4 und Aether. Es wird mit Wasserdamf destilliert.; | |
| With sodium hydroxide In ethanol; water for 1h; Heating; |
-
-
67-66-3
chloroform

-
-
90-05-1
2-methoxy-phenol

-
A
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
B
-
121-33-5
vanillin

| Conditions | Yield |
|---|---|
| With potassium carbonate | |
| With sodium hydroxide Man kann Vanillin und 2-Oxy-3-methoxy-benzaldehyd durch Wasserdampf von 1.5-2 Atm. druck trennen, mit dem ersteres schwerer als letzterer fluechtig ist; | |
| With sodium hydroxide In water at 20℃; for 24h; Reagent/catalyst; Reimer-Tiemann Phenol Formylation; Inert atmosphere; | A 10 %Chromat. B 58 %Chromat. |
| With sodium hydroxide at 50℃; for 6h; Reagent/catalyst; Temperature; Reimer-Tiemann Phenol Formylation; | A 38 %Chromat. B 33 %Chromat. |

-
-
90-05-1
2-methoxy-phenol

-
-
864131-95-3
N,N'-diphenylformamidine

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| at 210℃; und Kochen des Reaktionsproduktes mit Natronlauge; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 100℃; |
-
-
90-05-1
2-methoxy-phenol

-
-
91-78-1
1,3,5-triphenylhexahydro-1,3,5-triazine

-
A
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
B
-
121-33-5
vanillin

| Conditions | Yield |
|---|---|
| und Erhitzen der Reaktionsprodukte mit Nitrobenzol und wss.NaOH auf 120-130grad; |

-
-
67-66-3
chloroform

-
-
90-05-1
2-methoxy-phenol

-
A
-
621-59-0
isovanillin

-
B
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
C
-
121-33-5
vanillin

| Conditions | Yield |
|---|---|
| With potassium hydroxide; β‐cyclodextrin In water at 60℃; Yield given; | |
| With potassium hydroxide In water at 60℃; Product distribution; also catechol, var. amount BCD; |

-
-
128404-89-7
3-(2-(((2-hydroxy-3-methoxyphenyl)methylene)amino)-4-thiazolyl)-2H-1-benzopyran-2-one

-
-
79-04-9
chloroacetyl chloride

-
A
-
87503-74-0
2-chloro-N-(4-(2-oxo-2H-chromen-3-yl)thiazol-2-yl)acetamide

-
B
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| With triethylamine In 1,4-dioxane for 6h; Heating; Yield given; |


| Conditions | Yield |
|---|---|
| With n-butyllithium; boric acid tributyl ester; dihydrogen peroxide 1.) THP, 20 deg C, 60 min, 2.) 0 deg C, 1 h; Yield given. Multistep reaction; |
-
-
91-10-1
1,3-dimethoxy-2-hydroxy-benzene

-
A
-
934-00-9
3-methocycatechol

-
B
-
24677-78-9
2,3-dihydroxybenzaldehyde

-
C
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
D
-
95-48-7
ortho-cresol

| Conditions | Yield |
|---|---|
| at 500℃; Product distribution; Decomposition; |

-
-
225793-15-7
phenethyl 2,6-dimethoxyphenyl ether

-
A
-
824-42-0
2-methoxy-3-methylbenzaldehyde

-
B
-
91-10-1
1,3-dimethoxy-2-hydroxy-benzene

-
C
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
D
-
95-48-7
ortho-cresol

| Conditions | Yield |
|---|---|
| at 500℃; Product distribution; Thermodynamic data; Kinetics; Decomposition; |


-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| With potassium hydroxide; nitrobenzene at 120℃; |
-
-
67-66-3
chloroform

-
-
90-05-1
2-methoxy-phenol

-
A
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
B
-
121-33-5
vanillin

-
-
67-66-3
chloroform

-
-
90-05-1
2-methoxy-phenol

-
A
-
2931-90-0
5-formylvanillin

-
B
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
C
-
121-33-5
vanillin


-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde
-
-
100-44-7
benzyl chloride

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
2011-06-5
2-benzyloxy-3-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In methanol at 90℃; for 12h; | 100% |
| With potassium hydroxide In ethanol for 8h; Reflux; | 98% |
| With potassium hydroxide In ethanol for 12h; Heating; | 95% |
-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
106-94-5
propyl bromide

-
-
41828-10-8
3-methoxy-2-propoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 12h; Heating / reflux; | 100% |
| With potassium carbonate |
-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
106-95-6
allyl bromide

-
-
23343-06-8
2-allyloxy-3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 50℃; Williamson Ether Synthesis; | 100% |
| With sodium hydroxide; benzyltri(n-butyl)ammonium chloride In dichloromethane Ambient temperature; | 98% |
| With potassium carbonate In acetonitrile for 4h; Inert atmosphere; Reflux; | 98% |
-
-
67-52-7
BARBITURIC ACID

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
141266-45-7
1,5-Dihydro-9-methoxy-5-<5'-pyrimidine-2',4',6'(1'H,3'H,5'H)-trionyl>-2H-chromeno<2,3-d>pyrimidine-2,4(1H,3H)-dione

| Conditions | Yield |
|---|---|
| In ethanol at 100℃; for 0.5h; | 100% |
| In ethanol for 0.5h; Heating; | 74% |
-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
137898-13-6
2-trifluoromethylsulphonyloxy-3-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With dmap In pyridine Ambient temperature; | 100% |
| With pyridine In dichloromethane at 0℃; for 1h; | 95% |
| With pyridine In dichloromethane at 20℃; | 95% |
-
-
122476-70-4
3-(2-bromoacetyl)-4-hydroxy-1-methylquinolin-2(1H)-one

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
122476-74-8
4-hydroxy-3-(7-methoxybenzofuran-2-oyl)-1-methylquinolin-2-one

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol for 5h; Heating; | 100% |
-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
107-13-1
acrylonitrile

-
-
57543-69-8
8‐methoxy‐2H‐chromene‐3‐carbonitrile

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane for 20h; Heating; | 100% |
| With 1,4-diaza-bicyclo[2.2.2]octane In neat (no solvent) at 40℃; | 90% |
| With 1,4-diaza-bicyclo[2.2.2]octane In neat (no solvent) at 40℃; Morita-Baylis-Hillman Alkylation; | 90% |
-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
109-77-3
malononitrile

-
-
89770-21-8
2-(2-amino-3-cyano-8-methoxy-4H-<1>benzopyran-4-yl)propane-1,3-dinitrile

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate at 20℃; for 0.133333h; | 100% |
| With potassium fluoride In neat (no solvent) at 20℃; for 0.166667h; | 97% |
| In ethanol; water at 20℃; for 0.133333h; Irradiation; Green chemistry; | 96% |
-
-
174291-97-5
N-[(1S,2S)-2-aminocyclohexan-1-yl]-4-methylbenzenesulfonamide

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| In dichloromethane Ambient temperature; | 100% |
-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
953805-47-5
Naphthalene-1-sulfonic acid ((1S,2S)-2-amino-cyclohexyl)-amide

| Conditions | Yield |
|---|---|
| In dichloromethane Ambient temperature; | 100% |
-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
423761-68-6
(1S,2S)-N-(2,4,6-trimethylbenzenesulfonyl)-1,2-cyclohexanediamine

| Conditions | Yield |
|---|---|
| In dichloromethane Ambient temperature; | 100% |
-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| In dichloromethane Ambient temperature; | 100% |
-
-
106-49-0
p-toluidine

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
20772-64-9
2-methoxy-6-((E)-[(4-methylphenyl)imino]methyl)phenol

| Conditions | Yield |
|---|---|
| 100% | |
| In ethanol for 1h; Reflux; | 87% |
| With sodium sulfate In acetonitrile | |
| In ethanol Reflux; |
-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
75-26-3
isopropyl bromide

-
-
75792-35-7
2-isopropoxy-3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20 - 110℃; Industry scale; Inert atmosphere; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 110℃; | 99% |
| Stage #1: 3-methoxy-2-hydroxybenzaldehyde With tetrabutylammomium bromide; potassium carbonate In acetone at 20℃; for 0.25h; Stage #2: isopropyl bromide In acetone at 20℃; for 24h; | 96% |
-
-
2033-24-1
cycl-isopropylidene malonate

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
2555-20-6
8-methoxy-2-oxo-2H-chromene-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With piperdinium acetate In ethanol for 2h; Heating; | 100% |
| In water for 1h; Reflux; | 99% |
| With β‐cyclodextrin In water at 70℃; for 0.25h; Green chemistry; | 97% |
-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
109-77-3
malononitrile

-
-
13229-91-9
8-methoxy-2-oxo-2H-1-benzopyran-3-carbonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 3-methoxy-2-hydroxybenzaldehyde; malononitrile With sodium carbonate at 20℃; for 21h; Stage #2: With hydrogenchloride at 90℃; for 4h; Further stages.; | 100% |
| With zirconium(IV) chloride; 1-butyl-3-methylimidazolium Tetrafluoroborate at 25℃; for 0.583333h; Knoevenagel condensation; | 84% |
-
-
191480-63-4
(1R,2R)-1,2-diaminocyclohexane monohydrochloride

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| In methanol; ethanol at 20℃; for 18h; | 100% |
-
-
18668-68-3
4-bromo-buta-1,2-diene

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
1043422-10-1
2-(buta-2,3-dienyloxy)-3-methoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 17h; | 100% |
-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
654680-82-7
{(1R,3R,4S,6S)-4-amino-4,7,7-trimethylbicyclo[4.1.0]heptan-3-yl}methanol

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 24h; | 100% |
-
-
1236145-25-7, 69295-14-3
4-amino-1-(cyanomethyl)pyridinium chloride

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
1236145-52-0
1-(2-imino-8-methoxy-2H-chromen-3-yl)pyridin-4(1H)-iminium chloride

| Conditions | Yield |
|---|---|
| With sodium carbonate In water at 20℃; Knoevenagel condensation; | 100% |
-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
110-60-1
1,4-diaminobutane

-
-
104978-24-7
bis(2-hydroxy-3-methoxy-benzylidene)-tetramethylene-diamine

| Conditions | Yield |
|---|---|
| In methanol for 3h; Reflux; | 100% |
| In ethanol Reflux; |
-
-
7328-91-8
2,2-Dimethyl-1,3-diaminopropane

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
188482-90-8
N,N′-bis(3-methoxysalicylidene)-2,2-dimethyl-1,3-propanediamine

| Conditions | Yield |
|---|---|
| In methanol at 22℃; for 4h; | 100% |
| In ethanol for 1h; Reflux; | 92% |
| In ethanol for 1h; Reflux; | 92% |
-
-
5036-48-6
3-(1H-imidazol-1-yl)propan-1-amine

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
1334482-41-5
C15H19N3O2

| Conditions | Yield |
|---|---|
| In toluene for 5.58333h; Inert atmosphere; Reflux; | 100% |
-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
1427439-23-3
4-bromo-2-hydroxy-3-methoxy benzaldehyde

| Conditions | Yield |
|---|---|
| With sulfuric acid; C18H16Br4N2O3V; dihydrogen peroxide; potassium bromide In methanol; water at 20℃; for 0.166667h; Catalytic behavior; | 100% |
| With sulfuric acid; C20H22Br2N2O5V; dihydrogen peroxide In methanol; water at 20℃; for 0.25h; Catalytic behavior; | 100% |
-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
28144-70-9
anthranilic acid amide

-
-
1178895-46-9
2-[2-hydroxy-3-(methyloxy)phenyl]quinazolin-4(3H)-one

| Conditions | Yield |
|---|---|
| With copper(II) choride dihydrate In ethanol for 16h; Reflux; | 100% |
| With nickel diacetate In ethanol; dimethyl sulfoxide at 60 - 80℃; for 6h; | 90% |
| With yttrium(lll) nitrate hexahydrate In acetonitrile at 20℃; for 14h; | 88% |
| With copper(II) choride dihydrate In ethanol for 16h; Reflux; | |
| With copper(II) choride dihydrate In ethanol for 16h; Reflux; |
-
-
150-13-0
4-amino-benzoic acid

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
196617-29-5
4-{[(2-hydroxy-3-methoxyphenyl)methylidene]amino}benzoic acid

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol at 20℃; for 4h; | 100% |
| In ethanol; water at 20℃; for 12h; | 75% |
-
-
1236145-27-9
1-(cyanomethyl)-4-(ethoxycarbonylamino)pyridinium chloride

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
1236145-52-0
1-(2-imino-8-methoxy-2H-chromen-3-yl)pyridin-4(1H)-iminium chloride

| Conditions | Yield |
|---|---|
| With sodium carbonate at 20℃; for 1.5h; Green chemistry; | 100% |
-
-
1121-22-8, 1436-59-5, 20439-47-8, 21436-03-3, 41013-43-8, 694-83-7
trans-1,2-Diaminocyclohexane

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
177898-70-3
(S,S)-N,N′-bis-(3-methoxysalicylidene)cyclohexane-1,2-diamine

| Conditions | Yield |
|---|---|
| In methanol at 22℃; for 4h; | 100% |
-
-
57497-39-9
N-tertbutylhydroxylamine hydrochloride

-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| With pyrrolidine In dichloromethane at 20℃; for 0.15h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol Reflux; | 100% |
3-Methoxysalicylaldehyde Chemical Properties
Molecule structure of o-Vanillin (CAS NO.148-53-8):
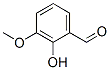
IUPAC Name: 2-Hydroxy-3-methoxybenzaldehyde
Molecular Weight: 152.14732 g/mol
Molecular Formula: C8H8O3
Density: 1.231 g/cm3
Melting Point: 40-42 °C(lit.)
Boiling Point: 265.5 °C at 760 mmHg
Flash Point: 94 °C
Index of Refraction: 1.587
Molar Refractivity: 41.56 cm3
Molar Volume: 123.5 cm3
Surface Tension: 47.3 dyne/cm
Enthalpy of Vaporization: 52.37 kJ/mol
Vapour Pressure: 0.00556 mmHg at 25 °C
Water Solubility: slightly soluble
Sensitive: air sensitive
XLogP3: 1.4
H-Bond Donor: 1
H-Bond Acceptor: 3
Rotatable Bond Count: 2
Tautomer Count: 5
Exact Mass: 152.047344
MonoIsotopic Mass: 152.047344
Topological Polar Surface Area: 46.5
Heavy Atom Count: 11
Canonical SMILES: COC1=CC=CC(=C1O)C=O
InChI: InChI=1S/C8H8O3/c1-11-7-4-2-3-6(5-9)8(7)10/h2-5,10H,1H3
InChIKey: JJVNINGBHGBWJH-UHFFFAOYSA-NEINECS: 205-715-3
Product Categories: Aromatic Aldehydes & Derivatives (substituted)
3-Methoxysalicylaldehyde History
In 1876, o-Vanillin was first isolated by renowned German chemist Ferdinand Tiemann. By 1910, methods for its purification had been developed by Francis Noelting, who similarly demonstrated its versatility as a general synthetic precursor for a diverse array of compounds, such as the coumarins. The compound began to show use as a dye for hides by 1920.
3-Methoxysalicylaldehyde Uses
o-Vanillin (CAS NO.148-53-8) can be used for the pharmaceutical and industries such as electroplating. Today, most o-Vanillin is used in the study of mutagenesis and as a synthetic precursor for pharmaceuticals.
3-Methoxysalicylaldehyde Toxicity Data With Reference
| 1. | skn-rbt 500 mg | FCTOD7 Food and Chemical Toxicology. 20 (1982),563. | ||
| 2. | eye-rbt 100 mg | FCTOD7 Food and Chemical Toxicology. 20 (1982),573. | ||
| 3. | eye-rbt 100 mg/4S rns MLD | FCTOD7 Food and Chemical Toxicology. 20 (1982),573. | ||
| 4. | ipr-rat LD50:347 mg/kg | COREAF Comptes Rendus Hebdomadaires des Seances de l’Academie des Sciences. 243 (1956),609. | ||
| 5. | orl-mus LD50:1330 mg/kg | IJANDP International Journal of Andrology. 10 (1987),741. | ||
| 6. | ipr-gpg LD50:400 mg/kg | COREAF Comptes Rendus Hebdomadaires des Seances de l’Academie des Sciences. 238 (1954),2576. |
3-Methoxysalicylaldehyde Consensus Reports
Reported in EPA TSCA Inventory.
3-Methoxysalicylaldehyde Safety Profile
Hazard Codes:  Xn,
Xn,  Xi
Xi
Risk Statements: 22-36/37/38
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 23-36-24/25-36/37/39-27-26
S23:Do not breathe vapour.
S36:Wear suitable protective clothing.
S24/25:Avoid contact with skin and eyes.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S27:Take off immediately all contaminated clothing.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
WGK Germany: 3
RTECS: CU6530000
F: 10-23
HS Code: 29124900
Poison by intraperitoneal route. An eye and skin irritant. When heated to decomposition it emits acrid smoke and irritating fumes. See also VANILLIN and ALDEHYDES.
3-Methoxysalicylaldehyde Specification
o-Vanillin (CAS NO.148-53-8) is also named as 2-Hydroxy-m-anisaldehyde ; 3-Methoxy-2-hydroxybenzaldehyde ; 3-Methoxysalicylaldehyde ; 4-08-00-01747 (Beilstein Handbook Reference) ; 6-Formyl-2-methoxyphenol ; 6-Formylguaiacol ; AI3-00076 ; BRN 0471913 ; CCRIS 8811 ; NSC 2150 ; O-Vanillin ; Orthovanilline ; Oxy-2 methoxy-3 benzaldehyde ; Oxy-2 methoxy-3 benzaldehyde [French] ; o-Vanilline . o-Vanillin (CAS NO.148-53-8) is a fibrous, light-yellow, crystalline solid.
Related Products
- 3-Methoxysalicylaldehyde
- 148546-82-1
- 148546-97-8
- 148546-99-0
- 148547-19-7
- 148553-50-8
- 148563-16-0
- 148565-57-5
- 1485-70-7
- 14857-34-2
- 14857-82-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View