-
Name
4-Chloroanisole
- EINECS 210-772-2
- CAS No. 623-12-1
- Article Data266
- CAS DataBase
- Density 1.137 g/cm3
- Solubility Immiscible with water
- Melting Point -18 °C
- Formula C7H7ClO
- Boiling Point 184.4 °C at 760 mmHg
- Molecular Weight 142.585
- Flash Point 72.3 °C
- Transport Information UN 2810
- Appearance colourless liquid
- Safety 23-24/25
- Risk Codes
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Anisole,p-chloro- (6CI,7CI,8CI);1-Chloro-4-methoxybenzene;1-Methoxy-4-chlorobenzene;4-Chlorophenol methyl ether;4-Methoxychlorobenzene;4-Methoxyphenyl chloride;Anisyl chloride;NSC 4129;p-Chloroanisole;p-Chloromethoxybenzene;p-Chlorophenyl methyl ether;p-Methoxychlorobenzene;p-Methoxyphenyl chloride;
- PSA 9.23000
- LogP 2.34860
Synthetic route
-
-
50619-99-3
4,4'-dimethoxy-diphenyliodonium chloride

-
-
623-12-1
4-chloromethoxybenzene

| Conditions | Yield |
|---|---|
| In benzene-d6 at 120℃; for 120h; Sealed tube; | 100% |

| Conditions | Yield |
|---|---|
| With nickel dichloride In N,N-dimethyl-formamide at 170℃; for 0.0833333h; microwave irradiation; | 99% |
| With trans-bis(glycinato)copper(II) monohydrate; tetramethlyammonium chloride In ethanol at 100℃; for 12h; Finkelstein Reaction; Schlenk technique; Inert atmosphere; | 91% |
| With copper(I) oxide; tetramethlyammonium chloride; L-proline In ethanol at 110℃; for 18h; Inert atmosphere; | 80% |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene at 90℃; for 16h; Product distribution; Further Variations:; Temperatures; Solvents; Pressures; reaction times; microwave irradiation; | 99% |
| With N,N'-dimethylimidazolium-2-carboxylate In acetonitrile at 160℃; for 2h; Microwave irradiation; Green chemistry; | 94% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In N,N-dimethyl-formamide for 0.0416667h; microwave irradiation; | 92% |

-
-
623-12-1
4-chloromethoxybenzene

| Conditions | Yield |
|---|---|
| With trichloroisocyanuric acid In water; ethyl acetate at 20℃; for 0.666667h; Open flask; | 98% |

| Conditions | Yield |
|---|---|
| With sodium chlorite; silica gel; manganese(III) acetylacetonate; sodium bromide In dichloromethane at 25℃; for 2h; | A 97% B 3% |
| With tetraethylammonium chloride; tetraethylammonium bromide Product distribution; 1.) CH2Cl2, RT, 1 h, electrolysis, 2.) CH2Cl2, RT, 24 h; other halogenating agents; other benzene derivatives and olefins; |

| Conditions | Yield |
|---|---|
| With methanol; Methyl formate; copper(l) chloride at 115℃; for 2h; Autoclave; Green chemistry; | 97% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; tetrabutylammomium bromide; potassium carbonate for 0.00694444h; Irradiation; microwave; | 89% |
| With sodium hydroxide at 100℃; | |
| With alkaline solution | |
| With potassium carbonate In acetone | |
| With lithium hydroxide In tetrahydrofuran for 1h; Ambient temperature; Yield given; |

| Conditions | Yield |
|---|---|
| With methanol; gold; hydrogen; caesium carbonate at 100℃; under 3800.26 Torr; for 71h; | 89% |
-
-
1330681-27-0
(4-methoxyphenyl)(mesityl)iodonium trifluoromethanesulfonate

-
-
623-12-1
4-chloromethoxybenzene

| Conditions | Yield |
|---|---|
| With copper(l) chloride In acetonitrile at 80℃; for 2h; | 87% |
-
-
14596-60-2
N-chloro-N-(4-methylphenyl)acetamide

-
-
100-66-3
methoxybenzene

-
A
-
623-12-1
4-chloromethoxybenzene

-
B
-
766-51-8
2-Chloroanisole

-
C
-
18931-78-7
N-(2-chloro-4-methylphenyl)acetamide

-
D
-
103-89-9
4-Methylacetanilide

| Conditions | Yield |
|---|---|
| at 110℃; for 12h; Mechanism; Orton Haloaniline Rearrangement; Inert atmosphere; Darkness; | A 82% B n/a C n/a D 87% |

-
-
138-24-9
phenyltrimethylammonium chloride

-
-
106-48-9
4-chloro-phenol

-
A
-
623-12-1
4-chloromethoxybenzene

-
B
-
121-69-7
N,N-dimethyl-aniline

| Conditions | Yield |
|---|---|
| With caesium carbonate In toluene for 6h; Heating; | A 85% B n/a |


| Conditions | Yield |
|---|---|
| With boron trifluoride In diethyl ether for 0.05h; microwave irradiation; | 85% |
-
-
623-12-1
4-chloromethoxybenzene

-
-
60633-25-2
2-bromo-4-chloroanisole

| Conditions | Yield |
|---|---|
| With (CH3)4Br In liquid sulphur dioxide at -23℃; Rate constant; | 100% |
| With (CH3)4Br In liquid sulphur dioxide at -23℃; | 100% |
| With iron(III) chloride; N-Bromosuccinimide; 1-butyl-3-methylimidazolium trifluoromethanesulfonimide In toluene regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With sodium; diphenyldisulfane In 1-methyl-pyrrolidin-2-one for 0.5h; Heating; | 100% |
| With calcium hydride; diphenyldisulfane In 1-methyl-pyrrolidin-2-one at 220℃; for 0.5h; | 99% |
| With copper(I) oxide; sodium methylate In methanol at 185℃; for 12h; Autoclave; | 96% |

| Conditions | Yield |
|---|---|
| With 2-(dicyclohexylphosphino)-4'-(N,N-dimethylamino)-1,1'-biphenyl at 100℃; Suzuki-Miyaura coupling; | 100% |
| With tris-(dibenzylideneacetone)dipalladium(0); caesium carbonate In tetrahydrofuran at 50℃; for 24h; Suzuki coupling; Inert atmosphere; | 100% |
| With 1,2-di-(N-2-methoxyethylbenzimidazoliummethyl)-benzene dichloride; potassium tert-butylate; palladium diacetate In water; N,N-dimethyl-formamide at 50℃; for 3h; Reagent/catalyst; Suzuki Coupling; | 100% |

| Conditions | Yield |
|---|---|
| With (55)Pd068(44)Ni032; potassium carbonate In water at 80℃; for 8h; Catalytic behavior; Buchwald-Hartwig Coupling; | 100% |
| With 2-dicyclohexylphosphino-2′,6′-diisopropoxybiphenyl; sodium t-butanolate In tetrahydrofuran at 80℃; for 2.5h; Reagent/catalyst; | 100% |
| With potassium tert-butylate; [(IPr)Pd(acac)Cl] In 1,2-dimethoxyethane at 50℃; for 4h; Buchwald-Hartwig coupling; | 99% |

| Conditions | Yield |
|---|---|
| With indium(III) chloride; trifluorormethanesulfonic acid In trifluoroacetic acid at 70℃; for 1.5h; | 100% |
-
-
623-12-1
4-chloromethoxybenzene

-
-
54960-98-4
2,4’,6-trimethoxybiphenyl

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); ruphos In tetrahydrofuran at 70℃; Negishi coupling; | 100% |
-
-
623-12-1
4-chloromethoxybenzene

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); ruphos In tetrahydrofuran at 70℃; for 15h; Negishi coupling; | 100% |
-
-
623-12-1
4-chloromethoxybenzene

-
-
121-97-1
4-Methoxypropiophenone

-
-
35258-41-4
1,2-bis-(4-methoxy-phenyl)-propan-1-one

| Conditions | Yield |
|---|---|
| With 2-methoxy-6-(N-methyl-N-phenyl-amino)phenyl(dicyclohexyl)phosphine; palladium diacetate; sodium t-butanolate In toluene at 110℃; for 18h; Schlenk technique; Inert atmosphere; | 100% |
| With PdCl2(C3H3N2(CH3))(C3H2N2(C6H3(C3H7)2)2); potassium tert-butylate In toluene at 80℃; for 6h; Inert atmosphere; | 89% |
| With C40H44ClN3Pd; sodium t-butanolate In toluene at 80℃; for 3h; Inert atmosphere; | 87% |

| Conditions | Yield |
|---|---|
| Stage #1: bromobenzene With lithium Stage #2: With zinc(II) chloride Stage #3: 4-chloromethoxybenzene; nickel phosphine amido pincer complex In tetrahydrofuran; 1-methyl-pyrrolidin-2-one at 70℃; for 12h; Negishi cross-coupling; Further stages.; | 99.2% |
| Stage #1: bromobenzene With iodine; magnesium In diethyl ether at 50℃; for 1h; Inert atmosphere; Stage #2: 4-chloromethoxybenzene With magnesium In diethyl ether at 45 - 50℃; for 2h; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| With cesium fluoride; tris-(dibenzylideneacetone)dipalladium(0) In 1,4-dioxane | 99% |
| With cesium fluoride; tris-(dibenzylideneacetone)dipalladium(0) In 1,4-dioxane | 99% |
| With tetrabutylammomium bromide; sodium methylate In water at 80℃; for 20h; Ullmann reaction; | 97% |

-
-
623-12-1
4-chloromethoxybenzene

| Conditions | Yield |
|---|---|
| With [IPr*·H][Pd(η3-cin)Cl2]; potassium tert-butylate at 60 - 80℃; for 2h; Buchwald-Hartwig Coupling; Sealed tube; | 99% |
| With potassium hydroxide; johnphos; 2-(di-t-butylphosphino)biphenyl palladium In toluene at 90℃; for 20h; | 94% |
| With [Pd(2-aminobiphenyl)(PiPr2ArXyl2)](OMs); sodium t-butanolate In tetrahydrofuran at 80℃; for 19h; Reagent/catalyst; Inert atmosphere; Schlenk technique; | 94% |
-
-
623-12-1
4-chloromethoxybenzene

| Conditions | Yield |
|---|---|
| With [IPr*·H][Pd(η3-cin)Cl2]; potassium tert-butylate at 60 - 80℃; for 2h; Buchwald-Hartwig Coupling; Sealed tube; | 99% |
| With (2-phenyl-1H-inden-3-yl)-dicyclohexylphosphinium tetrafluoroborate; bis(dibenzylideneacetone)-palladium(0); sodium t-butanolate In 1,2-dimethoxyethane at 20 - 120℃; Buchwald-Hartwig amination; Inert atmosphere; | 91% |
| With 1,1'-bis-(diphenylphosphino)ferrocene; bis(1,5-cyclooctadiene)nickel (0); sodium t-butanolate In toluene at 100℃; for 15h; | 88% |
| With bis(η3-allyl-μ-chloropalladium(II)); potassium tert-butylate; 1,3-bis[(2,6-diisopropyl)phenyl]imidazolinium chloride In 1,4-dioxane at 100℃; for 1.5h; Inert atmosphere; | 50% |
-
-
623-12-1
4-chloromethoxybenzene

-
-
100-61-8
N-methylaniline

-
-
55251-46-2
N-(4-methoxyphenyl)-N-methylaniline

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); di-tert-butylneopentylphosphonium tetrafluoroborate; sodium t-butanolate In toluene at 100℃; for 18h; Hartwig-Buchwald amination; | 99% |
| With dichloro(3-chloropyridinyl)(1,3-(diisopropylphenyl)-4,5-bis(dimethylamino)imidazol-2-ylidene)palladium(II); potassium tert-butylate In 1,4-dioxane at 80℃; for 18h; Reagent/catalyst; Solvent; Temperature; Buchwald-Hartwig Coupling; Inert atmosphere; Schlenk technique; Sealed tube; | 99% |
| With [IPr*·H][Pd(η3-cin)Cl2]; potassium tert-butylate at 60 - 80℃; for 2h; Buchwald-Hartwig Coupling; Sealed tube; | 99% |
-
-
292638-84-7
styrene

-
-
623-12-1
4-chloromethoxybenzene

-
-
1694-19-5
E-1-(phenyl)-2-(4'-methoxyphenyl)ethene

| Conditions | Yield |
|---|---|
| With tetrakis[μ-1-[(3-methoxyphenyl)methyl]-2-phenyl-3-[2-oxo-2-[(2-phenolato-kO)aminokN]ethyl]-1H-imidazoliumato-kC4]tetrapalladium; tetrabutylammomium bromide; sodium acetate at 140℃; for 12h; Heck Reaction; | 99% |
| With tetrabutylammonium acetate; palladium diacetate; DavePhos In 1,4-dioxane at 80℃; for 24h; Heck reaction; Inert atmosphere; Sealed tube; | 95% |
| With C128H58Cl2O2P4Pd; potassium carbonate In methanol at 60℃; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Heck Reaction; Inert atmosphere; Schlenk technique; | 93% |
-
-
623-12-1
4-chloromethoxybenzene

| Conditions | Yield |
|---|---|
| With monophosphine 1,2,3,4,5-pentaphenyl-1'-(di-tert-butylphosphino)ferrocene; bis(dibenzylideneacetone)-palladium(0); sodium t-butanolate In toluene at 80℃; for 12h; | 99% |
| With bis(η3-allyl-μ-chloropalladium(II)); 2,6-bis(2,4,6-triisopropylphenyl)phenyl(dicyclohexylphosphine); sodium t-butanolate In dodecane; toluene at 100℃; for 6h; Catalytic behavior; Reagent/catalyst; Glovebox; | 99% |
| With tri-tert-butyl phosphine; bis(dibenzylideneacetone)-palladium(0); sodium t-butanolate In toluene at 70℃; for 16h; | 97% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; C50H75ClP2Pd In tetrahydrofuran at 20℃; for 6h; Schlenk technique; Inert atmosphere; | 99% |
| With sodium t-butanolate; tris(dibenzylideneacetone)dipalladium(0) chloroform complex; 1,3,5,7-tetramethyl-8-phenyl-2,4,6-trioxa-8-phosphatricyclo[3.3.1.13,7]decane In toluene at 70℃; | 95% |
| With sodium t-butanolate In toluene at 100 - 110℃; for 24h; Buchwald-Hartwig amination; Inert atmosphere; Sealed vial; | 94% |
-
-
623-12-1
4-chloromethoxybenzene

-
-
53040-92-9
4-(4-tolyl)anisole

| Conditions | Yield |
|---|---|
| With potassium phosphate monohydrate; Ethyl 4-bromobenzoate; C52H49PRu; bis(dibenzylideneacetone)-palladium(0) In 1,4-dioxane at 100℃; for 3h; Suzuki-Miyaura Coupling; Inert atmosphere; | 99% |
| With potassium phosphate; C114H132Cl6N10Pd3 In water; isopropyl alcohol at 80℃; for 6h; Suzuki-Miyaura Coupling; Inert atmosphere; Schlenk technique; | 99% |
| With C58H82Cl4N6Pd2; potassium tert-butylate In water; isopropyl alcohol at 80℃; for 4h; Suzuki-Miyaura Coupling; Schlenk technique; Inert atmosphere; | 99% |
-
-
623-12-1
4-chloromethoxybenzene

-
-
446-53-7
2-fluorophenylmagnesium bromide

-
-
72093-47-1
4-methoxy-2'-fluorobiphenyl

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); N,N′-bis(2,6-diisopropylphenyl)imidazol-2-ylidene hydrochloride In tetrahydrofuran; 1,4-dioxane at 80℃; for 3h; Arylation; | 99% |
-
-
623-12-1
4-chloromethoxybenzene

-
-
4294-57-9
para-methylphenylmagnesium bromide

-
-
53040-92-9
4-(4-tolyl)anisole

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); N,N′-bis(2,6-diisopropylphenyl)imidazol-2-ylidene hydrochloride In tetrahydrofuran; 1,4-dioxane at 80℃; for 3h; Arylation; | 99% |
| With N-heterocyclic carbene-based nickel(II) complex In tetrahydrofuran at 20℃; for 12h; Kumada reaction; | 99% |
| With iron(II) triflate; 1,3-bis(2,4,6-trimethylphenyl)-4,5,6,7-tetrahydro-1H-1,3-diazepinium bromide; sodium t-butanolate In tetrahydrofuran at 60℃; for 16h; Kumada Cross-Coupling; Sealed tube; Inert atmosphere; | 99% |
-
-
623-12-1
4-chloromethoxybenzene

-
-
16419-60-6
2-Methylphenylboronic acid

-
-
92495-54-0
4'-methoxy-2-methylbiphenyl

| Conditions | Yield |
|---|---|
| With potassium fluoride; monophosphine 1,2,3,4,5-pentaphenyl-1'-(di-tert-butylphosphino)ferrocene; bis(dibenzylideneacetone)-palladium(0) In toluene at 80℃; for 72h; Suzuki coupling; | 99% |
| With potassium phosphate tribasic trihydrate; PdCl2(C3H3N2(CH3))(C3H2N2(C6H3(C3H7)2)2) In tetrahydrofuran; water at 20℃; for 24h; Suzuki-Miyaura coupling reaction; Inert atmosphere; | 99% |
| With potassium phosphate; C114H132Cl6N10Pd3 In water; isopropyl alcohol at 80℃; for 6h; Suzuki-Miyaura Coupling; Inert atmosphere; Schlenk technique; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-chloromethoxybenzene With nickel(II) acetylacetonate; bis(1-adamantyl)phosphine oxide In tetrahydrofuran at 20℃; for 0.0833333h; Stage #2: phenylmagnesium chloride In tetrahydrofuran at 20℃; Kumada cross-coupling; | 99% |
| With [{C4H2N-2,5-(CH2PPh2)2-κ3PNP}NiCl] In tetrahydrofuran; toluene at 23℃; for 12h; Kumada Cross-Coupling; Inert atmosphere; Schlenk technique; | 89% |
| With C61H78ClN4Ni2 In tetrahydrofuran at 20℃; for 18h; Reagent/catalyst; Kumada Cross-Coupling; Schlenk technique; | 85% |
-
-
623-12-1
4-chloromethoxybenzene

-
-
5720-07-0
4-methoxyphenylboronic acid

-
-
2132-80-1
4,4'-Dimethoxybiphenyl

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); bis(2,4-dimethoxyphenyl)phosphine oxide; cesium fluoride at 100℃; for 12h; | 99% |
| With potassium carbonate; palladium dichloride In various solvent(s) at 20℃; for 5h; Suzuki-Miyaura cross-coupling; | 98% |
| With potassium phosphate; 4-dicyclohexylphosphino-12-(2',6'-dimethoxy)phenyl-[2.2]paracyclophane; palladium diacetate In 1,4-dioxane at 80℃; for 12h; Suzuki-Miyaura coupling; Inert atmosphere; | 93% |
-
-
1013-88-3
Benzophenone imine

-
-
623-12-1
4-chloromethoxybenzene

-
-
42834-19-5
N-(4-methoxyphenyl)-1,1-diphenylmethanimine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; bis(dibenzylideneacetone)-palladium(0); 1,3-bis[2,6-diisopropylphenyl]imidazolium chloride In 1,4-dioxane at 100℃; for 6h; | 99% |
| With sodium t-butanolate In toluene at 100 - 110℃; for 48h; Buchwald-Hartwig amination; Inert atmosphere; Sealed vial; | 80% |

| Conditions | Yield |
|---|---|
| With [Pd(2-aminobiphenyl)(PCyp2ArXyl2)](OMs); sodium t-butanolate In tetrahydrofuran at 80℃; for 19h; Reagent/catalyst; Inert atmosphere; Schlenk technique; | 99% |
| With sodium t-butanolate; XPhos palladium(II) phenethylamine chloride In 1,4-dioxane at 100℃; for 0.166667h; | 98% |
| With C40H42NP; bis(dibenzylideneacetone)-palladium(0); sodium t-butanolate In 1,4-dioxane; water at 20℃; for 0.5h; Inert atmosphere; Sealed tube; Reflux; | 98% |
-
-
623-12-1
4-chloromethoxybenzene

-
-
75-66-1
2-methylpropan-2-thiol

-
-
7205-64-3
1-[(1,1-dimethylethyl)thio]-4-methoxybenzene

| Conditions | Yield |
|---|---|
| With sodium t-butanolate; palladium diacetate In 1,4-dioxane at 100℃; for 18h; | 99% |

| Conditions | Yield |
|---|---|
| With [Pd(2-aminobiphenyl)(PCyp2ArXyl2)](OMs); sodium t-butanolate In tetrahydrofuran at 80℃; for 19h; Reagent/catalyst; Inert atmosphere; Schlenk technique; | 99% |
| With tris-(dibenzylideneacetone)dipalladium(0); potassium carbonate; 2,8,9-tris(2-methylpropyl)-2,5,8,9-tetraaza-1-phosphabicyclo[3.3.3]undecane In toluene for 24h; Inert atmosphere; Reflux; | 72% |
| In 1,4-dioxane for 5h; |
-
-
623-12-1
4-chloromethoxybenzene

-
-
5720-06-9
2-Methoxyphenylboronic acid

-
-
49602-47-3
2,4'-dimethoxybiphenyl

| Conditions | Yield |
|---|---|
| With potassium phosphate; C46H52Cl3N3O2Pd In tetrahydrofuran; water at 60℃; for 4h; Suzuki-Miyaura Coupling; | 99% |
| With dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; potassium phosphate; water; palladium diacetate In tetrahydrofuran at 20℃; for 3h; Suzuki-Miyaura reaction; | 98% |
| With potassium phosphate; N,N'-bis-[2,4,6-tri(3,3,3-trifluoropropyl)phenyl]-4,5-dihydroimidazolium chloride; palladium diacetate In tetrahydrofuran; water at 20℃; for 12h; Suzuki-Miyaura cross-coupling; Inert atmosphere; | 95% |
| With [(N,N′-bis-(2,4,6-trimethylphenyl)imidazolidin-2-ylidene)PdCl2(pyrazole)]; sodium hydroxide In ethanol at 20℃; for 6h; Reagent/catalyst; Suzuki-Miyaura Coupling; Sealed tube; | 85% |
| With potassium phosphate; C28H31ClN2Ni; triphenylphosphine In tert-Amyl alcohol; toluene at 110℃; for 24h; Suzuki-Miyaura Coupling; Inert atmosphere; Sealed tube; | 81% |
4-Chloroanisole Chemical Properties
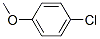
IUPAC Name:1-chloro-4-methoxybenzene
Molecular Formula:C7H7ClO
Molecular Weight:142.582880 g/mol
Appearance:yellow to colorless Clear liquid
Melting Point:-18 °C
Boiling Point:198-202 °C
Flash Point:173 °F
Solubility:ethanol and ether
Insolubility:water
Density:1.164 g/mL at 25 °C
Refractive index:n20/D 1.535
BRN:1858901
EINECS:210-772-2
Synonyms of 4-Chloroanisole (CAS NO.623-12-1): 1-chloro-4-methoxy-benzen ; 4-Chlorophenol methyl ether ; Anisole, p-chloro- ; Benzene,1-chloro-4-methoxy- ; para-Chloroanisole
Categories: Aromatic Ethers ; Anisoles, Alkyloxy Compounds & Phenylacetates ; Chlorine Compounds ; Ethers ; Organic Building Blocks ; Oxygen Compounds
4-Chloroanisole Uses
4-Chloroanisole (CAS NO.623-12-1) is used as an intermediate for fruit acid.
4-Chloroanisole Safety Profile
Safety Information of 4-Chloroanisole (CAS NO.623-12-1):
Hazard Codes:Xn 
Safety Statements:23-24/25
23:Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer)
24/25:Avoid contact with skin and eyes
RIDADR:UN 2810
WGK Germany:3
HS Code:29093090
4-Chloroanisole Specification
First Aid Measures of 4-Chloroanisole (CAS NO.623-12-1):
Eyes:Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids.
Skin:Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:Remove from exposure and move to fresh air immediately.
Storage of 4-Chloroanisole (CAS NO.623-12-1):
Store in a cool, dry place. Keep container closed when not in use.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View