-
Name
4-Nitroanisole
- EINECS 202-825-3
- CAS No. 100-17-4
- Article Data364
- CAS DataBase
- Density 1.222 g/cm3
- Solubility 0.468 g/L (20 °C) in water
- Melting Point 51-53 °C
- Formula C7H7NO3
- Boiling Point 260 °C at 760 mmHg
- Molecular Weight 153.137
- Flash Point 134.6 °C
- Transport Information UN 3458 6.1/PG 3
- Appearance Beige crystalline solid
- Safety 61-36/37
- Risk Codes 52/53-68
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Anisole,p-nitro- (8CI);1-Methoxy-4-nitrobenzene;4-Methoxy-1-nitrobenzene;4-Methoxynitrobenzene;4-Nitro-1-methoxybenzene;4-Nitroanisole;4-Nitromethoxybenzene;4-Nitrophenyl methyl ether;Methyl 4-nitrophenyl ether;Methyl p-nitrophenyl ether;NSC 5507;p-Methoxynitrobenzene;p-Nitroanisole;p-Nitrobenzene methyl ether;p-Nitromethoxybenzene;
- PSA 55.05000
- LogP 2.12660
Synthetic route

| Conditions | Yield |
|---|---|
| In various solvent(s) at 125℃; for 0.0583333h; microwave irradiation; | 100% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; sodium hydroxide In water at 65℃; pH=9.5; Temperature; Reagent/catalyst; | 99.01% |
| With poly(ethylene glycol) 400; sodium hydroxide at 110℃; for 8h; | 95% |
| With aluminum oxide; potassium hydroxide for 5h; microwave irradiation; | 89% |

| Conditions | Yield |
|---|---|
| In methanol at 40 - 60℃; for 1h; Temperature; Large scale; | 99% |
| With Amberlyst A27 In toluene at 65℃; for 4h; | 70% |
| [1,3-{bis-N-(N-methylimidazolylidene)methyl}-5-methylbenzenecopper dibromide] In ethyl acetate for 12h; Conversion of starting material; Heating / reflux; | 70% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide Ambient temperature; | 99% |
| Stage #1: 4-nitro-phenol With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetone for 0.166667h; Stage #2: methyl iodide In acetone at 20℃; for 4h; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 98% |

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride at 80℃; for 0.5h; | 99% |

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride at 80℃; | 98% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 70 - 80℃; for 2h; Large scale; | 97.2% |
| With sodium hydroxide at 70 - 80℃; for 2h; Large scale; | 97.2% |
| With ammonia; sodium hydroxide at 10 - 20℃; for 12h; Autoclave; | 97.5% |

| Conditions | Yield |
|---|---|
| With sodium nitrate In neat (no solvent) at 20℃; for 0.05h; Green chemistry; | 97% |
| With 3-methyl-1-sulfonic acid imidazolium nitrate In dichloromethane at 20℃; for 0.0166667h; | 92% |
| With bismuth(III) nitrate; Montmorillonite KSF for 0.2h; Nitration; | 91% |

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In 1,2-dichloro-ethane at 20℃; for 10h; | 95% |
| With 1,9-diperoxynonanedioic acid In acetonitrile at 50℃; for 0.5h; | 95% |
| With oxygen; methyltrioxorhenium(VII) In acetonitrile for 5h; Heating; | 92% |

| Conditions | Yield |
|---|---|
| With copper In 1-methyl-pyrrolidin-2-one at 250℃; for 0.25h; | 95% |
| With silver(I) acetate; potassium carbonate In 1-methyl-pyrrolidin-2-one at 120℃; for 16h; Inert atmosphere; | 88% |
| With copper(l) iodide; triethylamine In dimethyl sulfoxide at 120℃; under 760.051 Torr; for 20h; Inert atmosphere; Schlenk technique; | 85% |

| Conditions | Yield |
|---|---|
| Stage #1: methanol With N-benzyl-trimethylammonium hydroxide for 0.166667h; Neat (no solvent); Stage #2: 4-Fluoronitrobenzene at 20℃; for 0.166667h; Neat (no solvent); | 95% |
| With [(N,N′-bis(diisopropylphosphino)-2,6-diaminopyridine)Mn(CO)3][Br]; potassium hydroxide at 130℃; for 16h; Molecular sieve; Sealed tube; | 80% |
| With potassium hydroxide | |
| Stage #1: methanol With sodium hydride In tetrahydrofuran at 0℃; for 0.25h; Inert atmosphere; Stage #2: 4-Fluoronitrobenzene In tetrahydrofuran at 0 - 20℃; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; tetrabutylammonium nitrite; N,N`-dimethylethylenediamine In 1-methyl-pyrrolidin-2-one at 100℃; for 0.166667h; Microwave irradiation; Inert atmosphere; | 94% |
| With copper; tetrabutylammonium nitrite; N,N`-dimethylethylenediamine In N,N-dimethyl-formamide at 110℃; for 0.283333h; Inert atmosphere; Microwave irradiation; | 88% |
| With copper bronze; tetrabutylammonium nitrate; N,N`-dimethylethylenediamine In N,N-dimethyl-formamide at 100℃; for 3h; | 81% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In methanol; hexane; acetonitrile for 15h; Ambient temperature; | 93% |
-
-
403-66-7
p-nitrophenyl trifluoromethyl sulfide

-
-
124-41-4
sodium methylate

-
-
100-17-4
para-methoxynitrobenzene

| Conditions | Yield |
|---|---|
| In methanol for 2.5h; Heating; | 93% |

| Conditions | Yield |
|---|---|
| With diethylazodicarboxylate In tetrahydrofuran for 2h; | 93% |
| With boron trifluoride In diethyl ether for 0.05h; microwave irradiation; | 84% |

| Conditions | Yield |
|---|---|
| With magnesium oxide In N,N-dimethyl-formamide at 170℃; for 0.5h; Microwave irradiation; Green chemistry; | 93% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In N,N-dimethyl-formamide for 0.0416667h; microwave irradiation; | 90% |
| With tributyl-amine In N,N-dimethyl-formamide at 285℃; under 112511 Torr; for 0.05h; | 90% |
-
-
100-02-7
4-nitro-phenol

-
-
1087-09-8
1,4-diphenyl-but-2-yne-1,4-dione

-
-
121-45-9
phosphorous acid trimethyl ester

-
B
-
100-17-4
para-methoxynitrobenzene

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 24h; | A 86% B 92% |


| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); tert-butyl XPhos In N,N-dimethyl-formamide at 100℃; Inert atmosphere; | 92% |
-
-
117635-44-6
tert-butyldimethyl(4-nitrophenoxy)silane

-
-
74-88-4
methyl iodide

-
-
100-17-4
para-methoxynitrobenzene

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran Ambient temperature; | 91% |
-
-
733-53-9
p-methoxyphenyl(phenyl)iodonium tetrafluoroborate

-
A
-
591-50-4
iodobenzene

-
B
-
100-17-4
para-methoxynitrobenzene

-
C
-
696-62-8
para-iodoanisole

-
D
-
98-95-3
nitrobenzene

| Conditions | Yield |
|---|---|
| With sodium nitrite In chloroform; water at 56℃; for 3h; Title compound not separated from byproducts; | A 8 % Chromat. B 9% C 90% D 90 % Chromat. |
| With sodium nitrite In chloroform; water at 56℃; for 3h; | A 8 % Chromat. B 9% C 90% D 90 % Chromat. |
| With sodium nitrite In chloroform; water at 56℃; for 3h; Product distribution; other substituted arylphenyliodonium fluoroborates, diphenyliodonium fluoroborate, other reaction time; | A 8 % Chromat. B 9% C 90% D 90 % Chromat. |

-
-
1882-69-5
5-methoxy-2-nitro-benzoic acid

-
A
-
100-17-4
para-methoxynitrobenzene

-
B
-
124-38-9
carbon dioxide

| Conditions | Yield |
|---|---|
| With copper(I) oxide; bathophenanthroline In 1-methyl-pyrrolidin-2-one; quinoline at 170℃; for 16h; Product distribution; | A 90% B n/a |

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); caesium carbonate; tert-butyl XPhos In toluene at 85℃; for 0.42h; Inert atmosphere; | 90% |
| With caesium carbonate; copper(l) iodide at 110℃; for 12h; | 88% |
| With bis(μ-iodo)bis[(-)-sparteine]-dicopper(I); caesium carbonate at 120℃; for 12h; | 87% |

| Conditions | Yield |
|---|---|
| With 1,3-disulfonic acid imidazolium nitrate In neat (no solvent) at 20℃; for 0.0166667h; | 90% |
| With zirconium(IV) oxynitrate hydrate; iodine In toluene at 20℃; for 12h; Inert atmosphere; | 86% |
| With bismuth (III) nitrate pentahydrate In toluene at 70 - 80℃; for 2h; Inert atmosphere; | 85% |

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium carbonate at 150℃; for 2h; | 88% |
-
-
100-02-7
4-nitro-phenol

-
-
138-24-9
phenyltrimethylammonium chloride

-
A
-
100-17-4
para-methoxynitrobenzene

-
B
-
121-69-7
N,N-dimethyl-aniline

| Conditions | Yield |
|---|---|
| With caesium carbonate In toluene for 48h; Heating; | A 88% B n/a |

-
-
100-02-7
4-nitro-phenol

-
-
762-42-5
dimethyl acetylenedicarboxylate

-
-
121-45-9
phosphorous acid trimethyl ester

-
B
-
100-17-4
para-methoxynitrobenzene

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; | A 88% B n/a |

-
-
115298-63-0
phenyl(4-methoxyphenyl)iodonium triflate

-
A
-
100-17-4
para-methoxynitrobenzene

-
B
-
98-95-3
nitrobenzene

| Conditions | Yield |
|---|---|
| With sodium nitrite In ethyl acetate at 70℃; for 16h; Sealed tube; chemoselective reaction; | A 6% B 86% |

| Conditions | Yield |
|---|---|
| With silica supported Al(NO3)3*9H2O In acetone at 20℃; for 0.75h; regioselective reaction; | A 13% B 85% |
| With sodium nitrite for 0.0333333h; Reagent/catalyst; Microwave irradiation; | A 85% B 12% |
| With thionyl chloride; bismuth subnitrate In dichloromethane at 20℃; for 2h; | A 76% B 12% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In neat (no solvent) at 110℃; for 24h; Inert atmosphere; Sealed tube; | 85% |
| With caesium carbonate; copper(l) iodide at 110℃; for 12h; | 81% |
| With potassium phosphate; Cu(2,2'-bipyridyl)2BF4 at 80℃; for 24h; | 80% |

-
-
21090-30-2
3'-O-acetylthymidine

-
-
40615-39-2
5'-O-(4-4'-dimethoxytrityl)thymidine

-
A
-
100-17-4
para-methoxynitrobenzene

-
B
-
118149-31-8
5'-O-dimethoxytritylthimidine-3'-O-(5'-O-thymidylyl-3'-O-acetyl)phosphorothioate

| Conditions | Yield |
|---|---|
| Stage #1: O-methyl bis(O-4-nitrophenyl)phosphite; 5'-O-(4-4'-dimethoxytrityl)thymidine With sodium hydride In tetrahydrofuran at 20℃; for 0.5h; Inert atmosphere; Stage #2: 3'-O-acetylthymidine With sodium hydride In tetrahydrofuran at 20℃; for 2h; Inert atmosphere; Stage #3: With sulfur In diisopropylamine at 20℃; for 2h; Inert atmosphere; | A n/a B 85% |


| Conditions | Yield |
|---|---|
| With carbon monoxide; water; [Ru(cyclo-octa-1,5-diene)(pyridine)4][BPh4]2 In tetrahydrofuran at 170℃; for 20h; | 100% |
| With hydrogen In ethyl acetate at 20℃; under 7600.51 Torr; for 4h; chemoselective reaction; | 100% |
| With hydrogen In methanol at 20℃; for 2h; | 100% |
-
-
109-74-0
propyl cyanide

-
-
100-17-4
para-methoxynitrobenzene

-
A
-
61829-43-4
N-butyl-4-methoxyaniline

-
B
-
82749-62-0
N,N-dibutyl-4-methoxylaniline

| Conditions | Yield |
|---|---|
| Stage #1: para-methoxynitrobenzene With ammonium formate; palladium on activated charcoal In methanol; water at 20℃; Stage #2: propyl cyanide In methanol; water | A 100% B n/a |


| Conditions | Yield |
|---|---|
| With ammonium formate; palladium on activated charcoal In water; isopropyl alcohol at 20℃; for 1h; | 100% |
| With acetic acid; zinc In methanol at 20℃; for 0.5h; | 100% |

| Conditions | Yield |
|---|---|
| With hydrogen; sodium hydroxide In ethanol at 125℃; under 26252.6 Torr; for 4h; Concentration; Autoclave; | 99.3% |
| With hydrogen; palladium on activated charcoal In ethanol; N,N-dimethyl-formamide for 5h; | 79% |

| Conditions | Yield |
|---|---|
| Stage #1: para-methoxynitrobenzene With trichlorosilane; triethylamine In acetonitrile at 0 - 25℃; for 1.5h; Stage #2: acetic anhydride With methanol In acetonitrile at 25 - 65℃; for 24h; chemoselective reaction; | 99% |
| With indium; acetic acid In methanol at 20℃; for 1.5h; | 94% |
| Stage #1: para-methoxynitrobenzene With sodium tetrahydroborate In water at 20℃; for 0.5h; Green chemistry; Stage #2: acetic anhydride In water at 20℃; for 1.25h; Green chemistry; | 93% |
| With tin(ll) chloride |
-
-
100-17-4
para-methoxynitrobenzene

-
-
107-12-0
propiononitrile

-
A
-
107411-43-8
4-(4-methoxyphenyl)-dipropylamine

-
B
-
71193-47-0
N-(4-methoxyphenyl)-N-propylamine

| Conditions | Yield |
|---|---|
| Stage #1: para-methoxynitrobenzene With ammonium formate; palladium on activated charcoal In methanol; water at 20℃; Stage #2: propiononitrile In methanol; water | A n/a B 99% |


| Conditions | Yield |
|---|---|
| With hydrogen In toluene at 80℃; under 15001.5 Torr; for 18h; Autoclave; | 99% |
| With sodium tetrahydroborate In water at 20℃; for 0.583333h; Catalytic behavior; Green chemistry; | 96% |
| With 5% Au/Fe2O3; hydrogen In toluene at 120℃; under 15001.5 Torr; for 6h; Autoclave; | 95% |

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In ethyl acetate at 20℃; under 760.051 Torr; for 5h; | 99% |
| With 10% palladium on activated charcoal; hydrogen In tetrahydrofuran at 20℃; under 760.051 Torr; for 26h; | 84% |
-
-
100-17-4
para-methoxynitrobenzene

-
-
133745-75-2
N-fluorobis(benzenesulfon)imide

-
-
1453101-02-4
N-(2-methoxy-5-nitrophenyl)-N-(phenylsulfonyl)benzenesulfonamide

| Conditions | Yield |
|---|---|
| With [Ag(2,2'-bipyridine)2](ClO4); C20H28N4O2Pd*2CHF3O3S In acetonitrile at 23℃; for 24h; Inert atmosphere; Sealed tube; | 99% |
| With [Ag(2,2'-bipyridine)2](ClO4); C20H28N4O2Pd(2+)*2CF3O3S(1-) In acetonitrile at 23℃; for 24h; Inert atmosphere; Sealed tube; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: para-methoxynitrobenzene With proazaphosphatrane In tetrahydrofuran at 90℃; for 24h; Inert atmosphere; Schlenk technique; Stage #2: carbon dioxide at 90℃; under 750.075 Torr; for 1h; Inert atmosphere; Schlenk technique; | 99% |
| With Aminomethylphosphonic acid; water; 1-butyl-3-methylimidazolium trifluoromethanesulfonimide In acetonitrile at 30℃; under 750.075 Torr; for 10h; Electrochemical reaction; Saturated gas; | 74 %Spectr. |
-
-
100-17-4
para-methoxynitrobenzene

| Conditions | Yield |
|---|---|
| With [((B(C6F5)3)-1,3-bis(2,4,6-trimethylphenyl)imidazolin-2-ylidene)Ir(PPh2Me)(1,5-cyclooctadiene)]; deuterium In cyclohexane at 20℃; for 16h; Reagent/catalyst; Inert atmosphere; | 99% |
| With C33H50IrN3P(1+)*C32H12BF24(1-); deuterium In dichloromethane at 21 - 24℃; under 760.051 Torr; for 1h; |
-
-
100-17-4
para-methoxynitrobenzene

-
-
110-13-4
2,5-hexanedione

-
-
5044-27-9
1-(4-methoxyphenyl)-2,5-dimethyl-1H-pyrrole

| Conditions | Yield |
|---|---|
| With formic acid for 12h; Autoclave; Inert atmosphere; Green chemistry; | 98.9% |
| With indium; acetic acid In toluene at 80℃; for 1.5h; Inert atmosphere; | 95% |
| In tetrahydrofuran; water at 120℃; for 24h; | 94% |
| With carbon monoxide; water In tetrahydrofuran at 120℃; under 3800.26 Torr; for 24h; | 93% |

| Conditions | Yield |
|---|---|
| With trifluoromethylsulfonic anhydride; ethylammonium nitrate at 0 - 20℃; for 0.416667h; Inert atmosphere; regioselective reaction; | 98% |
| With nitric acid at 0℃; | |
| With bismuth (III) nitrate pentahydrate In 1,2-dichloro-ethane at 80 - 85℃; for 48h; | 94 %Chromat. |

| Conditions | Yield |
|---|---|
| With lithium chloride In N,N-dimethyl-formamide for 24h; Heating; | 98% |
| With water; hydrogen bromide; Aliquat 336 at 105℃; for 3.5h; Catalytic behavior; | 97% |
| With copper(I) oxide; sodium methylate In methanol at 185℃; for 12h; Autoclave; | 87% |

| Conditions | Yield |
|---|---|
| With silver nanoparticles decorated mesoporous 1,3,5-triformylphloroglucinol-DMB covalent organic framework nanomaterial In water at 20℃; for 2h; Catalytic behavior; Irradiation; Green chemistry; | 98% |
| With gold nano particles supported on rutile TiO2 In toluene at 70℃; under 750.075 Torr; for 3h; Inert atmosphere; chemoselective reaction; | 96% |
| With silver and palladium nanoparticles immobilized over the surface of graphitic carbon nitride (g-C3N4) In water for 2h; Irradiation; | 96% |
-
-
100-17-4
para-methoxynitrobenzene

-
-
16712-16-6, 64165-14-6, 65387-22-6, 70179-79-2, 540-69-2
ammonium formate

-
-
5470-34-8
4-methoxyformanilide

| Conditions | Yield |
|---|---|
| With cobalt nanoparticles coated by N,P-codoped carbon shell pyrolyzed at 800°C In tetrahydrofuran at 120℃; for 12h; Schlenk technique; Inert atmosphere; | 98% |
| With gold nanoparticles supported on titanium dioxide (TiO2) In acetonitrile for 2.5h; Inert atmosphere; Reflux; | 93% |
| With zinc for 0.0333333h; microwave irradiation; | 84% |
| With glycolic Acid at 30℃; for 48h; Irradiation; Inert atmosphere; | 17 %Spectr. |
-
-
100-17-4
para-methoxynitrobenzene

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
18437-68-8
tert-butyl N-(4-methoxyphenyl)carbamate

| Conditions | Yield |
|---|---|
| Stage #1: para-methoxynitrobenzene With sodium tetrahydroborate In water at 20℃; for 0.5h; Green chemistry; Stage #2: di-tert-butyl dicarbonate In water at 20℃; for 1.33333h; Green chemistry; | 98% |
-
-
14040-11-0
tungsten hexacarbonyl

-
-
100-17-4
para-methoxynitrobenzene

-
-
616-38-6
carbonic acid dimethyl ester

-
-
51-66-1
4-methoxyacetanilide

| Conditions | Yield |
|---|---|
| With di(rhodium)tetracarbonyl dichloride; 1,3-bis-(diphenylphosphino)propane; sodium phosphate; sodium iodide In water at 120℃; for 24h; Inert atmosphere; Sealed tube; | 98% |


| Conditions | Yield |
|---|---|
| With 0.5% Pd on alumina; hydrogen In ethyl acetate at 30℃; for 12h; Green chemistry; | 97.6% |
| With acetic acid; zinc In methanol at 20℃; for 0.5h; | 95% |
| With carbon monoxide; water In tetrahydrofuran at 120℃; under 3800.26 Torr; for 24h; | 74% |
-
-
100-17-4
para-methoxynitrobenzene

-
-
100-52-7
benzaldehyde

-
-
94664-76-3, 19064-76-7
(Z)-N-(4-methoxyphenyl)-1-phenylmethanimine oxide

| Conditions | Yield |
|---|---|
| With ammonium chloride; zinc In ethanol; water at 0 - 20℃; for 16h; | 97% |
| With ammonium chloride; zinc In ethanol; water at 0 - 20℃; | 63% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In water; dimethyl sulfoxide at 130℃; for 15h; Schlenk technique; Sealed tube; Green chemistry; | 97% |
| With acetic acid; zinc In methanol at 20℃; for 0.5h; | 96% |
| With methanol; hydrogen at 79.84℃; under 9750.98 Torr; for 1h; Autoclave; | |
| With formic acid; triethylamine In water; tert-butyl alcohol at 100℃; for 24h; Reagent/catalyst; | |
| With hydrogenchloride; iron In ethanol; water at 50℃; for 12h; | 28.2 g |
-
-
100-17-4
para-methoxynitrobenzene

-
-
104-88-1
4-chlorobenzaldehyde

-
-
20357-42-0
N-(4-chlorobenzyl)-4-methoxyaniline

| Conditions | Yield |
|---|---|
| With acetic acid; zinc In methanol at 20℃; for 0.5h; | 97% |
| With formic acid; triethylamine In water; tert-butyl alcohol at 100℃; for 24h; | 86% |
| With carbon monoxide; water In tetrahydrofuran at 120℃; under 3800.26 Torr; for 24h; | 80% |
4-Nitroanisole Consensus Reports
4-Nitroanisole Specification
The p-Nitroanisole with CAS registry number of 100-17-4 is also known as 4-Nitro-1-methoxybenzene. The IUPAC name is 1-Methoxy-4-nitrobenzene. It belongs to product categories of Aromatic Ethers. Its EINECS registry number is 202-825-3. In addition, the formula is C7H7NO3 and the molecular weight is 153.15. This chemical is a beige crystalline solid and soluble in alcohol, ether, boiling petroleum ether, slightly soluble in cold petroleum ether and insoluble in water. Besides, it should be sealed in ventilated, cool place away from oxidants, reducing agents, acids and alkali. This chemical is used as dye and pharmaceutical intermediates, mainly for the production of amino-anisole, blue salt, vitamin b.
Physical properties about p-Nitroanisole are: (1)ACD/LogP: 2.03; (2)ACD/LogD (pH 5.5): 2.03; (3)ACD/LogD (pH 7.4): 2.03; (4)#H bond acceptors: 4 #H bond donors: 0; (5)#Freely Rotating Bonds: 2; (6)Index of Refraction: 1.542; (7)Molar Refractivity: 39.47 cm3; (8)Molar Volume: 125.2 cm3; (9)Surface Tension: 42.2 dyne/cm; (10)Density: 1.222 g/cm3; (11)Flash Point: 134.6 °C; (12)Enthalpy of Vaporization: 47.76 kJ/mol; (13)Boiling Point: 260 °C at 760 mmHg; (14)Vapour Pressure: 0.0203 mmHg at 25 °C.
Preparation of p-Nitroanisole: it is prepared by reaction of 1-chloro-4-nitro-benzene with methanol; sodium salt. The reaction needs reagent amberlyst A27 and solvent toluene at the temperature of 60 °C. The yield is about 70%.
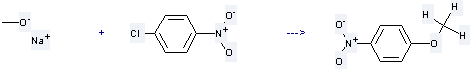
Uses of p-Nitroanisole: it is used to produce 2-iodo-4-nitro-anisole. The reaction occurs with reagent I2/F2 and solvents CHCl3, CCl3F at the temperature of 25 °C for 12 hours. The yield is about 80%.

When you are using this chemical, please be cautious about it. As a chemical, it is harmful to aquatic organisms that may cause long-term adverse effects in the aquatic environment. This chemical also has possible risk of irreversible effects. During using it, wear suitable protective clothing and gloves. Avoid release to the environment.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: COC1=CC=C(C=C1)[N+](=O)[O-]
2. InChI: InChI=1S/C7H7NO3/c1-11-7-4-2-6(3-5-7)8(9)10/h2-5H,1H3
3. InChIKey: BNUHAJGCKIQFGE-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 698mg/kg (698mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) | Toxicology and Applied Pharmacology. Vol. 41, Pg. 216, 1977. |
| mouse | LDLo | oral | 300mg/kg (300mg/kg) | Toksikologiya Novykh Promyshlennykh Khimicheskikh Veshchestv. Toxicology of New Industrial Chemical Substances. For English translation, see TNICS*. Vol. 4, Pg. 91, 1962. | |
| rat | LD50 | intraperitoneal | 1400mg/kg (1400mg/kg) | BEHAVIORAL: GENERAL ANESTHETIC GASTROINTESTINAL: OTHER CHANGES LIVER: FATTY LIVER DEGERATION | Archiv fuer Gewerbepathologie und Gewerbehygiene. Vol. 17, Pg. 217, 1959. |
| rat | LD50 | oral | 2300mg/kg (2300mg/kg) | Trudy Leningradskogo Sanitarno-Gigienicheskogo Meditsinskogo Instituta. Vol. 128, Pg. 14, 1979. | |
| rat | LD50 | skin | > 16gm/kg (16000mg/kg) | LIVER: FATTY LIVER DEGERATION KIDNEY, URETER, AND BLADDER: OTHER CHANGES | Archiv fuer Gewerbepathologie und Gewerbehygiene. Vol. 17, Pg. 217, 1959. |
Related Products
- 4-Nitroanisole
- 10017-44-4
- 1001754-72-8
- 1001754-82-0
- 1001757-50-1
- 10017-66-0
- 100189-17-1
- 1001906-63-3
- 1001907-59-0
- 1001907-60-3
- 1001907-65-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View