-
Name
5-Bromouracil
- EINECS 200-084-0
- CAS No. 51-20-7
- Article Data61
- CAS DataBase
- Density 1.965 g/cm3
- Solubility soluble in cold water
- Melting Point >300 °C(lit.)
- Formula C4H3BrN2O2
- Boiling Point 384oC
- Molecular Weight 190.984
- Flash Point
- Transport Information
- Appearance White powder.
- Safety 36/37-53-45
- Risk Codes 22-46
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 2,4 (1H, 3H)-Pyrimidinedione, 5-bromo-;5-Bromo-2,4-dihydroxypyrimidine;Bromouracil;5-bromo-1H-pyrimidine-2,4-dione;Uracil, 5-bromo-;5-Bromo-pyrimidin-2,4-diol;2,4-dihydroxy-5-Bromo-pyrimidine;5-Bromouracil;5-Bromo uracil;5-bromopyrimidine-2,4-diol;
- PSA 65.72000
- LogP -0.17430
Synthetic route

| Conditions | Yield |
|---|---|
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; acetic anhydride; acetic acid at 50℃; for 1.5h; Temperature; Reagent/catalyst; | 99.9% |
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; acetic anhydride; acetic acid at 50℃; for 1.5h; Temperature; Reagent/catalyst; | 99.9% |
| With N-Bromosuccinimide; montmorillonite K-10; tetrabutylammomium bromide for 0.0666667h; microwave irradiation; | 96% |
-
-
97759-31-4
5,5'-mercuribis(uracil)

-
-
51-20-7
5-bromouracil

| Conditions | Yield |
|---|---|
| With bromine; potassium bromide In water at 80℃; for 0.5h; | 84% |
-
-
664988-37-8
5-bromo-2,4-bis(4-methoxybenzyloxy)pyrimidine

-
-
51-20-7
5-bromouracil

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane at 20℃; for 0.25h; | 84% |
-
-
56686-16-9
5-bromo-2,4-dimethoxypyrimidine

-
-
51-20-7
5-bromouracil

| Conditions | Yield |
|---|---|
| With hydrogenchloride | 83% |

-
-
51-20-7
5-bromouracil

| Conditions | Yield |
|---|---|
| With bromine; potassium bromide In water; acetonitrile at 80℃; for 0.5h; | 77% |

| Conditions | Yield |
|---|---|
| With Bromoform In dichloromethane for 40h; Irradiation; | A 7% B 53% |

| Conditions | Yield |
|---|---|
| With bromine In water for 7h; Heating; | 40% |
-
-
22384-59-4
3-(3,4-dihydro-5-bromo-2,4-dioxo-(2H)pyrimidin-1-yl)propanoic acid methyl ester

-
-
141-43-5
ethanolamine

-
A
-
51-20-7
5-bromouracil

-
B
-
1174386-67-4
3-(5-bromo-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-N-(2-hydroxyethyl)-propanamide

-
C
-
1174386-68-5
N-(2-hydroxyethyl)-3-[5-(2-hydroxyethylamino)-2,4-dioxo-3,4-dihydro(2H)pyrimidin-1-yl]propanamide

| Conditions | Yield |
|---|---|
| In methanol for 12h; Reflux; | A 32% B 30% C 35% |


| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane at 20℃; | A 30% B 20% |

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane at 20℃; | A 30% B 18% |

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane at 20℃; | A 30% B 21% |

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane at 20℃; | A 30% B 22% |

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane at 20℃; | A 30% B 20% |
-
-
1124-83-0
5,5-dibromo-6-hydroxy-5,6-dihydrouracil

-
A
-
51-20-7
5-bromouracil

-
B
-
476-61-9
[5,5']bipyrimidinyl-2,4,6,2',4',6'-hexaone

| Conditions | Yield |
|---|---|
| With water |

-
-
51-20-7
5-bromouracil

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
15018-62-9
5-bromo-2,6-dioxo-1,2,3,6-tetrahydropyrimidine-4-carboxylic acid

-
-
51-20-7
5-bromouracil

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane; water at 25℃; for 1h; Title compound not separated from byproducts; | A 17 % Spectr. B 73 % Spectr. |

-
-
51-20-7
5-bromouracil

| Conditions | Yield |
|---|---|
| With hydroxylamine at 25℃; Equilibrium constant; var. pH; |
-
-
7647-01-0
hydrogenchloride

-
-
1124-83-0
5,5-dibromo-6-hydroxy-5,6-dihydrouracil

-
-
7732-18-5
water

-
-
51-20-7
5-bromouracil

| Conditions | Yield |
|---|---|
| at 25℃; Rate constant; |


-
-
51-20-7
5-bromouracil

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

-
-
51-20-7
5-bromouracil

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With hydrogen bromide; bromine |

| Conditions | Yield |
|---|---|
| Mechanism; |

-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

-
-
7783-56-4
antimony(III) fluoride

-
A
-
51-21-8
5-fluorouracil

-
B
-
51-20-7
5-bromouracil


| Conditions | Yield |
|---|---|
| In dimethylsulfoxide-d6; water Kinetics; byproducts: 5-bromo-6-hydroxy-5,6-dihydrouracil; reduction reaction at 25°C; monitored by NMR-spectroscopy; | |
| In dimethylsulfoxide-d6 Kinetics; reduction reaction at 25°C; monitored by (1)H-NMR-spectroscopy; |


| Conditions | Yield |
|---|---|
| With potassium dihydrogenphosphate; E-pyrimidine nucleoside phosphorylase-0002 In aq. buffer at 40℃; for 0.666667h; pH=9; Equilibrium constant; Thermodynamic data; Temperature; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With potassium dihydrogenphosphate; E-pyrimidine nucleoside phosphorylase-0002 In aq. buffer at 40℃; for 0.666667h; pH=9; Equilibrium constant; Thermodynamic data; Temperature; Enzymatic reaction; |
-
-
109-01-3
1-methyl-piperazine

-
-
51-20-7
5-bromouracil

-
-
141692-30-0
5-(4-methylpiperazin-1-yl) pyrimidine-2,4(1H,3H)-dione

| Conditions | Yield |
|---|---|
| With pyridine at 150℃; for 0.75h; Microwave irradiation; | 100% |
| In neat (no solvent) at 120℃; Microwave irradiation; | 91% |
| for 0.25h; Heating; | 65% |
-
-
51-20-7
5-bromouracil

-
-
78907-31-0
C4H4BrN2O2

| Conditions | Yield |
|---|---|
| With H2SO4 glasses Product distribution; Irradiation; | 100% |
-
-
51-20-7
5-bromouracil

-
-
36082-50-5
2,4-dichloro-5-bromopyrimidine

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride In 1,1,2-trichloroethane Reagent/catalyst; Solvent; Reflux; | 99.5% |
| With N,N-dimethyl-aniline; trichlorophosphate at 120 - 130℃; for 1.33333h; | 96.6% |
| With phosgene; Tributylphosphine oxide at -5 - 125℃; for 1.83333h; Inert atmosphere; | 89.7% |
-
-
75-77-4
chloro-trimethyl-silane

-
-
51-20-7
5-bromouracil

-
-
33282-64-3
2,4-bis-(trimethylsilyloxy)-5-bromopyrimidine

| Conditions | Yield |
|---|---|
| With 1,1,1,3,3,3-hexamethyl-disilazane Ambient temperature; | 99% |
| With 1,1,1,3,3,3-hexamethyl-disilazane at 140 - 150℃; for 5h; | |
| With 1,1,1,3,3,3-hexamethyl-disilazane | |
| With ammonium sulfate; 1,1,1,3,3,3-hexamethyl-disilazane for 18h; Heating; |

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 60℃; Aza-Micheal reaction; | 99% |
| With tetrabutylammomium bromide; zinc(II) oxide at 100℃; for 0.416667h; Michael addition; microwave irradiation; | 90% |
| With tetrabutylammomium bromide at 100℃; for 0.416667h; Microwave irradiation; Neat (no solvent); | 84% |
| With Bacillus subtilis alkaline protease EC 3.4.21.14 In dimethyl sulfoxide at 50℃; for 24h; Michael addition; | 68.5% |

| Conditions | Yield |
|---|---|
| at 110℃; for 0.116667h; microwave irradiation; | 98% |
-
-
51-20-7
5-bromouracil

-
-
292638-85-8
acrylic acid methyl ester

-
-
22384-59-4
3-(3,4-dihydro-5-bromo-2,4-dioxo-(2H)pyrimidin-1-yl)propanoic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 5-bromouracil With triethylamine In N,N-dimethyl-formamide for 0.0833333h; Michael-type addition; Stage #2: acrylic acid methyl ester In N,N-dimethyl-formamide at 20℃; for 24h; Michael-type addition; regioselective reaction; | 98% |
| With triethylamine In N,N-dimethyl-formamide at 60℃; Aza-Micheal reaction; | 97% |
| With acylase Amano from Aspergillus oryzae In dimethyl sulfoxide at 50℃; for 3h; Michael addition reaction; | 91 % Chromat. |
| With triethylamine In N,N-dimethyl-formamide at 20℃; for 24h; Michael condensation; |
-
-
51-20-7
5-bromouracil

-
-
141-32-2
acrylic acid n-butyl ester

-
-
1370413-76-5
3-(5-bromo-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)propionic acid butyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 60℃; Aza-Micheal reaction; | 98% |
-
-
51-20-7
5-bromouracil

-
-
103-67-3
benzyl-methyl-amine

-
-
141692-27-5
5-[benzyl(methyl)amino]-1H-pyrimidine-2,4-dione

| Conditions | Yield |
|---|---|
| at 120℃; for 0.0833333h; microwave irradiation; | 97% |
| In neat (no solvent) at 120℃; for 1h; Microwave irradiation; | 86% |
| at 120℃; for 1h; Microwave irradiation; | 86% |
| at 120℃; for 1h; Microwave irradiation; | 86% |
| for 0.25h; Heating; |

| Conditions | Yield |
|---|---|
| With oxygen; ozone In acetic acid at 20℃; for 0.75h; Product distribution; | 97% |

| Conditions | Yield |
|---|---|
| at 90℃; for 0.0833333h; microwave irradiation; | 96% |
| at 130℃; for 0.25h; | 96% |
| at 100℃; for 0.166667h; Microwave irradiation; | 89% |
| In neat (no solvent) at 120℃; Microwave irradiation; | 78% |

| Conditions | Yield |
|---|---|
| at 90℃; for 0.166667h; microwave irradiation; | 96% |

| Conditions | Yield |
|---|---|
| In ethanol for 24h; Reflux; | 96% |
-
-
51-20-7
5-bromouracil

-
-
72812-45-4
5-Bromo-4-thioxo-3,4-dihydro-1H-pyrimidin-2-one

| Conditions | Yield |
|---|---|
| With diphosphorus pentasulfide; sodium hydrogencarbonate In diethylene glycol dimethyl ether at 110℃; for 2h; | 95% |
-
-
51-20-7
5-bromouracil

-
-
100-46-9
benzylamine

-
-
28485-19-0
5-(benzylamino)dihydropyrimidine-2,4(1H,3H)-dione

| Conditions | Yield |
|---|---|
| at 110℃; for 0.2h; microwave irradiation; | 95% |
| at 160℃; for 2h; | 90% |
| for 0.666667h; Inert atmosphere; Heating; neat (no solvent); | 84% |
| Heating; Yield given; | |
| at 160℃; for 3h; |

| Conditions | Yield |
|---|---|
| at 90℃; for 0.15h; microwave irradiation; | 95% |
-
-
51-20-7
5-bromouracil

-
-
2177-18-6
vinyl acrylate

-
-
1217794-07-4
3-(5-bromouracil-1-yl)propionic acid vinyl ester

| Conditions | Yield |
|---|---|
| With D-aminoacylase from Escherichia coli In dimethyl sulfoxide at 25℃; for 0.416667h; Michael addition; Enzymatic reaction; regioselective reaction; | 95% |
-
-
51-20-7
5-bromouracil

-
-
105-36-2
ethyl bromoacetate

-
-
381710-01-6
5-bromo-3,4-dihydro-2,4-dioxo-1(2H)-pyrimidineacetic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 5-bromouracil With 1,1,1,3,3,3-hexamethyl-disilazane Heating; Stage #2: ethyl bromoacetate In acetonitrile Heating; Further stages.; | 94% |
| With ammonium sulfate; 1,1,1,3,3,3-hexamethyl-disilazane; potassium iodide In acetonitrile at 120℃; for 1h; Time; Hilbert-Johnson Synthesis; Microwave irradiation; Autoclave; regioselective reaction; | 62% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 2h; | 55% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; |
-
-
848644-37-1
1,2,3,5-tetra-O-acetyl-3-C-ethynyl-D-ribo-pentofuranose

-
-
51-20-7
5-bromouracil

-
-
848644-42-8
1-[2,3,5-tri-O-acetyl-3-C-ethynyl-β-D-ribo-pentofuranosyl]-5-bromouracil

| Conditions | Yield |
|---|---|
| With N,O-bis-(trimethylsilyl)-acetamide; trimethylsilyl trifluoromethanesulfonate In acetonitrile at 50℃; for 23h; | 94% |
-
-
51-20-7
5-bromouracil

-
-
89-99-6
2-fluorobenzylamine

-
-
867151-45-9
5-[(2-fluorobenzyl)amino]dihydropyrimidine-2,4(1H,3H)-dione

| Conditions | Yield |
|---|---|
| at 160℃; for 2h; | 94% |

| Conditions | Yield |
|---|---|
| With N,N-dimethyl acetamide at 180℃; for 7h; | 93.8% |
| With isopropyl alcohol Product distribution; Mechanism; Quantum yield; Irradiation; deuterated 2-propanol solvents; | |
| In ethanol Product distribution; Quantum yield; Mechanism; Irradiation; further solvent; |
-
-
51-20-7
5-bromouracil

-
-
72235-52-0
2,4-Difluoro-benzylamine

-
-
867151-48-2
5-[(2,4-difluorobenzyl)amino]dihydropyrimidine-2,4(1H,3H)-dione

| Conditions | Yield |
|---|---|
| at 160℃; for 6h; | 93% |
| at 160℃; for 2h; | 91% |
-
-
51-20-7
5-bromouracil

-
-
1585-16-6
2-chloromethyl-1,3,5-trimethylbenzene

-
-
1056025-50-3
5-bromo-1-(mesitylmethyl)pyrimidine-2,4(1H,3H)-dione

| Conditions | Yield |
|---|---|
| Stage #1: 5-bromouracil With benzenesulfonamide In acetonitrile for 0.25h; Stage #2: 2-chloromethyl-1,3,5-trimethylbenzene In acetonitrile for 20h; Heating; Further stages.; | 93% |

| Conditions | Yield |
|---|---|
| at 180℃; for 0.25h; microwave irradiation; | 92% |
| at 195 - 200℃; for 1h; | 77% |
| In ethylene glycol Reflux; | 68% |
| With hydroquinone In ethylene glycol at 200℃; for 2h; Heating / reflux; | 55% |
-
-
51-20-7
5-bromouracil

-
-
140-75-0
para-fluorobenzylamine

-
-
867151-47-1
5-[(4-fluorobenzyl)amino]dihydropyrimidine-2,4(1H,3H)-dione

| Conditions | Yield |
|---|---|
| at 160℃; for 2h; | 92% |

| Conditions | Yield |
|---|---|
| With zinc(II) oxide at 130℃; for 0.5h; Ionic liquid; | 92% |
-
-
51-20-7
5-bromouracil

-
-
64-04-0
phenethylamine

-
-
25912-34-9
5-[(2-phenylethyl)amino]dihydropyrimidine-2,4(1H,3H)-dione

| Conditions | Yield |
|---|---|
| at 160℃; for 2h; | 91% |

| Conditions | Yield |
|---|---|
| In ethanol at 100℃; for 16h; | 90.4% |
| With potassium carbonate In tetrahydrofuran |
-
-
51-20-7
5-bromouracil

-
-
187990-50-7
ethyl 6-O-(tert-butyldimethylsilyl)-4-O-methoxycarbonyl-2,3-dideoxy-α-D-erythro-hex-2-enopyranoside

| Conditions | Yield |
|---|---|
| With 1,4-di(diphenylphosphino)-butane; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran at 50℃; for 2h; | 90% |
5-Bromouracil Chemical Properties
Structure of 5-Bromouracil (CAS NO.51-20-7):
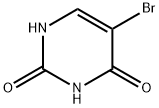
IUPAC Name: 5-bromo-1H-pyrimidine-2,4-dione
Empirical Formula: C4H3BrN2O2
Molecular Weight: 190.9828
EINECS: 200-084-0
Index of Refraction: 1.59
Molar Refractivity: 32.78 cm3
Molar Volume: 97.1 cm3
Polarizability: 12.99×10-24cm3
Surface Tension: 54.6 dyne/cm
Density: 1.965 g/cm3
Melting Point: >300 °C(lit.)
Physical Appearance: White powder
Water Solubility: SOLUBLE IN COLD WATER
Product Categories: Pyrimidine series;Detergents;Biochemistry;Nucleobases and their analogs;Nucleosides, Nucleotides & Related Reagents;Nucleic acids;Bases & Related Reagents;Mutagenesis Research Chemicals;Nucleotides
Synonyms of 5-Bromouracil (CAS NO.51-20-7): 5-Bromo-2,4(1H,3H)-pyrimidinedione ; Bromouracil ; 2,4(1H,3H)-Pyrimidinedione, 5-bromo- ; Uracil, 5-bromo-
5-Bromouracil Uses
A major chemical mutagen. Incorporates into DNA, altering base-pair sequencing by replacing thymine.
5-Bromouracil Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 1400mg/kg (1400mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: MUSCLE WEAKNESS LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Proceedings of the Society for Experimental Biology and Medicine. Vol. 93, Pg. 124, 1956. |
| rat | LD50 | intraperitoneal | 1700mg/kg (1700mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: MUSCLE WEAKNESS LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Proceedings of the Society for Experimental Biology and Medicine. Vol. 93, Pg. 124, 1956. |
5-Bromouracil Safety Profile
Hazard Codes:  Xn
Xn
Risk Statements: 22-46
R22:Harmful if swallowed.
R46:May cause heritable genetic damage.
Safety Statements: 36/37-53-45
S36/37:Wear suitable protective clothing and gloves.
S53:Avoid exposure - obtain special instructions before use.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
WGK Germany: 3
RTECS: YQ9060000
HS Code: 29335995
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View