-
Name
Benzylamine
- EINECS 202-854-1
- CAS No. 100-46-9
- Article Data960
- CAS DataBase
- Density 0.979 g/cm3
- Solubility Soluble in water
- Melting Point -30 °C
- Formula C7H9N
- Boiling Point 185 °C at 760 mmHg
- Molecular Weight 107.155
- Flash Point 60 °C
- Transport Information UN 2735 8/PG 2
- Appearance colourless liquid with an ammoniacal odour
- Safety 26-36/37/39-45
- Risk Codes 21/22-34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms phenylmethanamine;1-phenylmethanamine;Monobenzylamine;.omega.-Aminotoluene;benzylazanium;(Phenylmethyl)amine;benzenemethanamine;.alpha.-Aminotoluene;Benzylamine(BA);Benzyl amine;N-Benzylamine;Benzylamine 99%;
- PSA 26.02000
- LogP 1.84560
Synthetic route

| Conditions | Yield |
|---|---|
| With Zn(BH4)2(Ph3P)2 In tetrahydrofuran for 0.25h; Reduction; Heating; | 100% |
| With sodium tetrahydroborate; tin bis(1,2-benzenedithiolate) In tetrahydrofuran; phosphate buffer at 10℃; for 0.5h; pH=10; Product distribution; Further Variations:; pH-values; Solvents; Reduction; | 100% |
| With (Sn(SPh)3)(Et3N) In benzene at 15℃; for 0.0833333h; | 99% |

| Conditions | Yield |
|---|---|
| With lithium borohydride; 9-methoxy-9-BBN In diethyl ether at 25℃; for 5h; Product distribution; rate of reduction; | 100% |
| With borane N-ethyl-N-isopropylaniline complex In tetrahydrofuran for 0.1h; Heating; | 100% |
| With hydrogen; palladium In methanol at 20℃; for 432h; | 100% |
-
-
104669-74-1
benzyl carbamic acid allyl ester

-
-
100-46-9
benzylamine

| Conditions | Yield |
|---|---|
| With diethylamine; palladium diacetate; trisodium tris(3-sulfophenyl)phosphine In water; acetonitrile for 0.166667h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With Thiosalicylic acid; 1,4-di(diphenylphosphino)-butane; bis(dibenzylideneacetone)-palladium(0) In tetrahydrofuran at 60℃; for 0.5h; effect of temperature on deprotection of various primary and secondary allylamines; | 100% |
| With Thiosalicylic acid; 1,4-di(diphenylphosphino)-butane; bis(dibenzylideneacetone)-palladium(0) In tetrahydrofuran at 60℃; for 0.5h; | 100% |
| With polymethylhydrosiloxane; zinc(II) chloride; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran at 20℃; | 92% |
| With 1,3-bis[(diphenylphosphino)propane]dichloronickel(II); diisobutylaluminium hydride In toluene for 1h; Ambient temperature; | 66% |

| Conditions | Yield |
|---|---|
| With Thiosalicylic acid; 1,4-di(diphenylphosphino)-butane; bis(dibenzylideneacetone)-palladium(0) In tetrahydrofuran at 60℃; for 0.5h; | 100% |
| With 1,3-bis[(diphenylphosphino)propane]dichloronickel(II); diisobutylaluminium hydride In toluene for 1h; Ambient temperature; | 79% |
-
-
42116-44-9
N-tert-butoxycarbonylbenzylamine

-
-
100-46-9
benzylamine

| Conditions | Yield |
|---|---|
| With methanol; Acetyl bromide In dichloromethane at 25℃; for 0.333333h; | 100% |
| With 3-butyl-l-methyl-1H-imidazol-3-iumtrifloroacetate In 1,4-dioxane; water at 80 - 82℃; for 4h; | 98% |
| With nitric acid In dichloromethane at 0℃; for 1h; | 95% |

| Conditions | Yield |
|---|---|
| In aq. phosphate buffer for 0.5h; pH=7.4; | A n/a B 100% C n/a |

-
-
52826-45-6
(benzylimino)triphenylphosphorane

-
-
100-46-9
benzylamine

| Conditions | Yield |
|---|---|
| With phenylsilane In toluene at 111℃; for 12h; | 100% |

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen In methanol at 90℃; under 15001.5 Torr; for 4h; Solvent; Temperature; Pressure; Autoclave; | 99.7% |
| With Candida boidinii formate dehydrogenase; Geobacillus stearothermophilus ε‐deaminating L‐lysine dehydrogenase variant 1; nicotinamide adenine dinucleotide In aq. buffer at 30℃; for 24h; pH=8.5; Reagent/catalyst; Enzymatic reaction; | 99% |
| With ammonium hydroxide; hydrogen In ethanol at 130℃; under 7500.75 Torr; for 12h; Autoclave; | 96.9% |

| Conditions | Yield |
|---|---|
| Stage #1: N-benzyl-p-toluenesulfonamide With n-butyllithium In tetrahydrofuran at 0℃; Stage #2: With naphthalene; lithium In tetrahydrofuran at -78 - 20℃; | 99% |
| Stage #1: N-benzyl-p-toluenesulfonamide With n-butyllithium In tetrahydrofuran at 0℃; for 0.166667h; Inert atmosphere; Stage #2: With naphthalene; lithium In tetrahydrofuran at -78 - 25℃; Stage #3: With water In tetrahydrofuran | 99% |
| With naphthalene; water; lithium 1.) THF, -78 deg C, 2 h; Yield given. Multistep reaction; |
-
-
383865-57-4
4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl-amine

-
B
-
100-46-9
benzylamine

| Conditions | Yield |
|---|---|
| A 99% B n/a |

| Conditions | Yield |
|---|---|
| With sodium ethanolate In methanol at 80℃; for 2h; | 99% |
| With sodiumsulfide nonahydrate In water at 100℃; for 12h; | 86% |
| Multi-step reaction with 2 steps 1: 3 h / Reflux 2: triphenylphosphine; triethylamine / dichloromethane; tetrachloromethane / 4 h / Reflux; Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| With 5%-palladium/activated carbon; hydrogen; ammonium formate In methanol under 2068.65 Torr; Flow reactor; | 98% |
| With palladium on activated charcoal; tetrabutylammomium bromide; water; sodium hydroxide; silicon at 100℃; for 6h; Reagent/catalyst; | 94% |
| With hydrazine hydrate In dichloromethane at 20℃; for 1h; | 71% |
-
-
122954-29-4
benzylammonium O-ethylstyrylphosphonate

-
A
-
5849-49-0
styrylphosphonic acid ethylester

-
B
-
100-46-9
benzylamine

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 20℃; for 0.5h; | A 98% B 83% |

| Conditions | Yield |
|---|---|
| With ammonium cerium (IV) nitrate; water; acetic acid In dichloromethane at 20℃; for 7.5h; Inert atmosphere; | 98% |
| With water; ytterbium(III) triflate In tetrahydrofuran at 20℃; Product distribution; Further Variations:; Catalysts; Reagents; Hydrolysis; | 93% |
-
-
54535-21-6
2-(benzylamino-methylene)-malonic acid diethyl ester

-
-
100-46-9
benzylamine

| Conditions | Yield |
|---|---|
| With ethylenediamine In ethanol at 20℃; for 0.75h; | 98% |

-
-
100-46-9
benzylamine

| Conditions | Yield |
|---|---|
| Stage #1: N-benzyl-3,3-dimethoxypropylsulfonamide With p-toluenesulfonic acid monohydrate In water; acetone at 0 - 20℃; for 6h; Stage #2: With sodium hydroxide In methanol; water; acetone at 0 - 20℃; for 1.08333h; | 98% |

| Conditions | Yield |
|---|---|
| With sodium hydrogensulfate monohydrate; molybdenum(V) chloride; sodium cyanoborohydride In N,N-dimethyl-formamide for 1.5h; Reflux; | 96% |
| With sodium tetrahydroborate at 20℃; for 0.0333333h; neat (no solvent, solid phase); | 94% |
| With iron oxide; zirconium(IV) chloride; sodium cyanoborohydride In neat (no solvent) at 75 - 80℃; for 0.25h; Reagent/catalyst; Temperature; | 93% |

| Conditions | Yield |
|---|---|
| With sodium azide; triphenylphosphine In dichloromethane; N,N-dimethyl-formamide at 90℃; for 4h; Substitution; Mitsunobu reaction; Staudinger reaction; | 96% |
| With ammonia In toluene at 110℃; under 5250.53 Torr; for 20h; | 91% |
| With (carbonyl)(chloro)(hydrido)tris(triphenylphosphine)ruthenium(II); ammonia; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In tert-Amyl alcohol at 140℃; for 20h; Inert atmosphere; Cooling; | 87% |

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal; ethylenediamine In tetrahydrofuran at 20℃; for 24h; atmospheric pressure; | A 96% B n/a |

| Conditions | Yield |
|---|---|
| With 5-methyl-1,3,4-thiadiazol-2-amine; triethylamine In ethanol; water at 25℃; for 1h; | 96% |
| Multi-step reaction with 2 steps 1.1: n-butyllithium / tetrahydrofuran / Inert atmosphere 1.2: 8 h / Inert atmosphere 1.3: 2 h / 60 °C / Inert atmosphere 2.1: titanium(III) chloride; water / tetrahydrofuran / pH 10 / Reflux; Alkaline aq. solution; Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: potassium carbonate / N,N-dimethyl-formamide / 3 h / 80 °C 2: hydrogenchloride / water / 3 h / 100 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: potassium carbonate / 1 h / Milling 1.2: 1 h / Milling 2.1: ethylenediamine / neat (no solvent) / Milling View Scheme |

| Conditions | Yield |
|---|---|
| With C25H19BrMnN2O2P; potassium tert-butylate; hydrogen In toluene at 130℃; under 15001.5 Torr; for 48h; | A 98 %Spectr. B 96% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 20℃; for 0.25h; | 95% |

| Conditions | Yield |
|---|---|
| at 220℃; under 10 Torr; for 0.0833333h; Product distribution; pyrolysis without solvent, isolated as sulfate; | A n/a B 95% |

-
-
100-46-9
benzylamine

| Conditions | Yield |
|---|---|
| With ammonium formate; 1,2-bis-(diphenylphosphino)ethane; bis(dibenzylideneacetone)-palladium(0) In dimethyl sulfoxide at 80℃; for 12h; deprotection; | 95% |

| Conditions | Yield |
|---|---|
| With ammonium bromide; 3-azapentane-1,5-diamine at 110℃; for 5h; Temperature; Microwave irradiation; | 95% |

| Conditions | Yield |
|---|---|
| With [RuCl2(2-(diphenylphosphino)-N-((6-((diphenylphosphino)methyl)pyridin-2-yl)methyl)ethan-1-amine)]; potassium tert-butylate; hydrogen In tetrahydrofuran at 100℃; under 37503.8 Torr; for 20h; Catalytic behavior; Autoclave; chemoselective reaction; | A 95% B 87% |
| With C25H19BrMnN2O2P; potassium tert-butylate; hydrogen In tetrahydrofuran at 130℃; under 22502.3 Torr; for 48h; Mechanism; Inert atmosphere; Glovebox; Autoclave; Green chemistry; | A 94% B 92% |
| With [Ru(PtBuNNHtBu)H(CO)Cl]; potassium tert-butylate; hydrogen In tetrahydrofuran at 110℃; under 15001.5 Torr; for 24h; Autoclave; | |
| With C25H19BrMnN2O2P; potassium tert-butylate; hydrogen In 1,4-dioxane at 130℃; under 22502.3 Torr; for 48h; Catalytic behavior; Solvent; Reagent/catalyst; Pressure; Inert atmosphere; Glovebox; Autoclave; Green chemistry; | A 92 %Spectr. B 97 %Spectr. |

| Conditions | Yield |
|---|---|
| With copper(ll) sulfate pentahydrate; ammonium hydroxide In PEG1000-DIL; methyl cyclohexane at 60℃; for 3h; | 94% |
| With 5-methyl-1,3,4-thiadiazol-2-amine; triethylamine In ethanol; water at 25℃; for 1h; Reagent/catalyst; | 94% |
| With hydrogenchloride; potassium hydride; 1,1,3,3-tetramethyldisilazane In tetrahydrofuran at 0 - 25℃; multistep selective monoamination reaction of various alkyl halides; | 88% |
-
-
119592-74-4
N-benzyl-2,2,6,6-tetramethyl-2,6-disilapiperidine

-
-
100-46-9
benzylamine

| Conditions | Yield |
|---|---|
| In hydrogenchloride for 8h; Heating; | 94% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 20℃; | 100% |
| In dichloromethane at 0 - 20℃; | 100% |
| In dichloromethane at 0 - 20℃; | 100% |

| Conditions | Yield |
|---|---|
| With bis(trifluoromethane)sulfonimide lithium In chloroform at 85℃; for 40h; | 100% |
| Stage #1: benzylamine With diisobutylaluminium hydride In tetrahydrofuran; toluene Stage #2: 4-butanolide In tetrahydrofuran at 20℃; for 0.5h; | 98% |
| In benzene for 12h; Reflux; | 98% |

| Conditions | Yield |
|---|---|
| With copper(II) bis(trifluoromethanesulfonate) In water at 20℃; for 0.0166667h; | 100% |
| In water at 20℃; for 2h; | 93% |
| In dichloromethane Inert atmosphere; Molecular sieve; | 81% |
-
-
286-20-4
cyclohexane-1,2-epoxide

-
-
100-46-9
benzylamine

-
-
40571-86-6, 40571-87-7, 51925-39-4, 131164-07-3, 141553-09-5
rac-(1R,2R)-2-(benzylamino)cyclohexanol

| Conditions | Yield |
|---|---|
| bismuth(lll) trifluoromethanesulfonate at 160℃; for 0.25h; microwave irradiation; | 100% |
| at 150℃; Neat (no solvent); | 99% |
| With zinc(II) perchlorate hexahydrate at 80℃; for 1h; | 97% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 25℃; for 1h; | 100% |
| With triethylamine In dichloromethane at 0 - 20℃; for 4h; Inert atmosphere; | 100% |
| With 1-methyl-1H-imidazole In dichloromethane at 0℃; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With formic acid; Cp*IrCl(N-(phenyl(pyridin-2-yl)methyl)methanesulfonamide)complex In ethyl acetate at 40℃; for 18h; Reagent/catalyst; Inert atmosphere; | 100% |
| Stage #1: cyclohexanone; benzylamine With formic acid; chlorido(8-quinolinolato-k2N,O)(η5-pentamethylcyclopentadienyl)iridium(III) In ethyl acetate at 0 - 40℃; Inert atmosphere; Schlenk tube; Cooling with ice; Stage #2: With sodium hydrogencarbonate In water; ethyl acetate Product distribution / selectivity; | 98% |
| With 4 A molecular sieve; borane pyridine complex In methanol for 16h; | 96% |

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 1h; | 100% |
| for 6h; Kinetics; Molecular sieve; Reflux; | 100% |
| With magnesium sulfate In dichloromethane at 20℃; Inert atmosphere; | 65% |
-
-
104-88-1
4-chlorobenzaldehyde

-
-
100-46-9
benzylamine

-
-
130517-96-3, 13540-93-7
N-(4-chlorobenzylidene)benzylamine

| Conditions | Yield |
|---|---|
| for 6h; Molecular sieve; Reflux; | 100% |
| In ethanol at 20℃; | 94% |
| In dichloromethane at 20℃; for 16h; Molecular sieve; | 92% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |
| In methanol at 20℃; | 80% |

| Conditions | Yield |
|---|---|
| In hexane at 20℃; for 2h; | 100% |
| In dichloromethane at 20℃; | 98% |
| In chloroform for 0.5h; Heating; | 88% |

| Conditions | Yield |
|---|---|
| In toluene at 120℃; for 24h; | 100% |
| With magnesium sulfate In dichloromethane for 3h; Reflux; | 100% |
| With aluminum oxide at 20℃; for 2.5h; | 99% |

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 1h; | 100% |
| With sodium sulfate In dichloromethane at 20℃; | 99% |
| In chloroform at 20℃; for 1h; | 96.8% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; | 100% |
| With sodium hydroxide In 1,2-dimethoxyethane; water at 25℃; for 0.5h; pH 10.45; | 99% |
| Stage #1: benzoyl chloride; benzylamine With triethylamine In dichloromethane at 20℃; for 0.333333h; Stage #2: With poly{trans-bicyclo[2.2.1]hept-5-ene-2,3-di(chlorocarbonyl)} In dichloromethane at 20℃; for 0.5h; | 99% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 3h; | 100% |
| In acetonitrile at 25℃; for 0.166667h; Milling; | 99% |
| With C64H52CaN6 In neat (no solvent) at 60℃; for 12h; Schlenk technique; Glovebox; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With Iron(III) nitrate nonahydrate In toluene for 2h; Concentration; Reflux; | 100% |
| With H-β-zeolite In neat (no solvent) at 80℃; for 24h; Green chemistry; | 99% |
| at 20 - 120℃; for 15h; | 99% |

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 20℃; Inert atmosphere; | 100% |
| With aluminum oxide at 20℃; for 7h; | 90% |
| In water Condensation; | 65.12% |
-
-
292638-85-8
acrylic acid methyl ester

-
-
100-46-9
benzylamine

-
-
23574-01-8
3-benzylamino-propionic acid methyl ester

| Conditions | Yield |
|---|---|
| at -40℃; for 12h; | 100% |
| With [HP(HNCH2CH2)3N]NO3 In acetonitrile at 20℃; for 48h; Michael addition; | 98% |
| copper In methanol at 20℃; for 0.3h; Aza-Michael Addition; | 98% |
-
-
292638-85-8
acrylic acid methyl ester

-
-
100-46-9
benzylamine

-
-
793-19-1
bis(2-methoxycarbonylethyl)benzylamine

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 8h; Reflux; | 100% |
| In methanol Reflux; | 99% |
| In methanol at 34℃; for 72h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| In dichloromethane | 100% |
| With triethylamine In dichloromethane at 0 - 20℃; for 4h; Inert atmosphere; | 98% |
| With potassium carbonate In dichloromethane Heating; | 97% |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 2h; | 100% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 16h; | 99% |
| In dichloromethane at 0 - 20℃; for 0.5h; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane 1) 0 deg C, 1 h, 2) room temperature; | 100% |
| With sodium hydroxide In water at 25℃; for 2h; pH 9.6; | 99.9% |
| With Fe3O4-supported (diisopropylamino)acetamide In dichloromethane at 25℃; | 98% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 4℃; | 100% |
| With pyridine; dmap In dichloromethane at 0 - 20℃; for 16h; | 99% |
| With triethylamine at 20℃; for 1h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 10 - 35℃; for 16h; | 100% |
| In dichloromethane at 5℃; for 0.5h; | 99% |
| Multistep reaction; | 96% |

| Conditions | Yield |
|---|---|
| at 100℃; for 12h; Ionic liquid; Green chemistry; | 100% |
| aluminum oxide; zinc(II) oxide at 100℃; for 2h; Condensation; | 98% |
| In water at 20℃; for 1.33333h; Solvent; Time; Green chemistry; | 97% |

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 288h; | 100% |
| In dichloromethane for 13h; Inert atmosphere; Reflux; | 98.3% |
| In dichloromethane for 13h; Reflux; Inert atmosphere; | 95.3% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; ethyl cyanoglyoxylate-2-oxime In water; N,N-dimethyl-formamide at 20℃; for 3h; Reagent/catalyst; Solvent; | 100% |
| With 4-methyl-morpholine; dmap; 2-chloro-4,6-dimethoxy-1 ,3,5-triazine In dichloromethane at 35℃; for 0.1h; microwave irradiation; | 99% |
| With sulfated tungstate at 70℃; for 0.166667h; Neat (no solvent); | 99% |
-
-
54221-37-3
diethyl meso-2,5-dibromoadipate

-
-
100-46-9
benzylamine

-
-
17740-40-8
diethyl meso-1-benzyl-2,5-pyrrolidinedicarboxylate

| Conditions | Yield |
|---|---|
| In toluene for 4h; Reflux; | 100% |
-
-
97-65-4
2-methylenesuccinic acid

-
-
100-46-9
benzylamine

-
-
5733-86-8
1-benzyl-5-oxopyrrolidine-3-carboxylic acid

| Conditions | Yield |
|---|---|
| at 130℃; Inert atmosphere; | 100% |
| 86% | |
| at 130℃; for 2.5h; Inert atmosphere; | 82.9% |
Benzylamine Chemical Properties
Molecular Structure of Benzylamine (CAS NO.100-46-9):
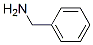
IUPAC Name: phenylmethanamine
Molecular formula: C7H9N
Molecular Weight:107.15
H bond acceptors: 1
H bond donors: 2
Freely Rotating Bonds: 2
Polar Surface Area: 3.24 Å2
Index of Refraction: 1.547
Molar Refractivity: 34.7 cm3
Molar Volume: 109.4 cm3
Surface Tension: 38.8 dyne/cm
Density: 0.979 g/cm3
Flash Point: 60 °C
Enthalpy of Vaporization: 42.12 kJ/mol
Boiling Point: 185 °C at 760 mmHg
Vapour Pressure: 0.713 mmHg at 25°C
EINECS: 202-854-1
Melting point: -30 °C
Product Categories: Pharmaceutical Intermediates; Organics
InChI
InChI=1/C7H9N/c8-6-7-4-2-1-3-5-7/h1-5H,6,8H2
Smiles
c1(ccccc1)CN
Benzylamine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mammal (species unspecified) | LD50 | oral | 700mg/kg (700mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 39(4), Pg. 86, 1974. | |
| mouse | LD50 | intraperitoneal | 600mg/kg (600mg/kg) | United States Patent Document. Vol. #3816470, |
Benzylamine Safety Profile
The Hazard Codes:  C
C
The Risk Statements information:
34: Causes burns
21/22: Harmful in contact with skin and if swallowed
The Safety Statements information:
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
36/37/39: Wear suitable protective clothing, gloves and eye/face protection
RIDADR: UN 2735 8/PG 2
Benzylamine Specification
Benzylamine , with CAS number of 100-46-9, can be called .alpha.-Aminotoluene ; (Aminomethyl)benzene ; (Phenylmethyl)amine ; Benzenemethanamine ; Moringine ; alpha-Aminotoluene . Benzylamine (CAS NO.100-46-9) consists of a benzyl group, attached to an amine functional group. This colorless liquid is a common precursor in organic synthesis.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View