-
Name
Calcium hydride
- EINECS 232-189-2
- CAS No. 7789-78-8
- Article Data108
- CAS DataBase
- Density 1.9
- Solubility Soluble in water and alcohol. Insoluble in benzene.
- Melting Point 190 °C(lit.)
- Formula CaH2
- Boiling Point 1484oC
- Molecular Weight 42.0939
- Flash Point
- Transport Information UN 1404 4.3/PG 1
- Appearance greyish-white solid
- Safety 24/25-43-7/8-43A
- Risk Codes 15
-
Molecular Structure
-
Hazard Symbols
 F
F
- Synonyms Calcium hydride (CaH2);Calciumdihydride;
- PSA 0.00000
- LogP 0.22500
Synthetic route

| Conditions | Yield |
|---|---|
| In melt High Pressure; Ca melted in vac. at 350°C for 5 h, filled with H2 (5 atm) at 400°C for 5 min, at 10 atm. at 480°; elem. anal.; | 99.6% |
| In neat (no solvent) hydrogenation in a closed iron tube under pressure at temperatures between 650°C and 690°C;; | 90% |
| In neat (no solvent) hydrogenation in a closed iron tube under pressure at temperatures between 650°C and 690°C;; | 90% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) heating the mixture in H2-atmosphere to 420-600°C;; product-mixture;; | |
| In neat (no solvent) heating the mixture in H2-atmosphere to 420-600°C;; product-mixture;; |


| Conditions | Yield |
|---|---|
| With ammonia at red heat; | |
| With NH3 at red heat; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) on evacn. of system(metal, loaded (Ar) into reactor-autoclave of the hydrogenation apparatus)at 300-350°C (0.5-1h),cooling to room temp. and feeding hydrogen into reactor (from LaNi5 "accumulator") to give pressure of 2.8MPa, 150°C, 1h;; on gas-volumetric and chemical anal.; XRD (orthorhombic, lattice parameters); react. without an induction period and forming a clinker;; | |
| In neat (no solvent) on evacn. of system(metal, loaded (Ar) into reactor-autoclave of the hydrogenation apparatus)at 300-350°C (0.5-1h),cooling to room temp. and feeding hydrogen into reactor (from LaNi5 "accumulator") to give pressure of 3.5MPa, 200°C, 1h;; on gas-volumetric and chemical anal.; XRD (orthorhombic, lattice parameters); react. is running without an induction period and forming of a clinker;; |

| Conditions | Yield |
|---|---|
| at dark red heat; |


| Conditions | Yield |
|---|---|
| byproducts: H2, N2; vigorous reaction at 400°C; | |
| byproducts: H2, N2; vigorous reaction at 400°C; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: calcium oxide; bombarding solid CaCl2 deposited on substrate with atomar H for 60 mins in plasmochemical app. described by I. Sh. Normatov, N. Shermatov, and U. Mirsaidov, Fiz. Khim. Obrab. Mater., No. 3, 141-142 (1990);; formation of metallic layer, containing oxide phase of calcium; X-ray diffraction;; | A 0% B n/a |


| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: HCl; bombarding periodically stirred CaCl2 (on substrate) with H for 120 mins in plasmochemical app. described by I. Sh. Normatov, N. Shermatov, and U. Mirsaidov, Fiz. Khim. Obrab. Mater., No. 3, 141-142 (1990); grinding; bombarding with H for 120 mins;; X-ray diffraction, DTA, IR spectroscopy;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) reaction of CaCl2 with H2 formed by dissociation of CH4;; |


| Conditions | Yield |
|---|---|
| reversible react.; | |
| at dark red heat and at temperatures above it; |


| Conditions | Yield |
|---|---|
| With hydrogen at red heat; | |
| With hydrogen | A n/a B 0% |
| With H2 | A n/a B 0% |
| With H2 at red heat; |
-
-
74-85-1
ethene

-
-
7440-70-2
calcium

-
A
-
7789-78-8
calcium hydride

-
B
-
75-20-7
calcium carbide

-
C
-
7440-44-0
pyrographite

| Conditions | Yield |
|---|---|
| In neat (no solvent) dark red heat; crust of CaC2, C and CaH2 inhibit reaction;; | |
| In neat (no solvent) dark red heat; crust of CaC2, C and CaH2 inhibit reaction;; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: H2; 375-390°C; no isolation, monitoring by thermal anal.; |

| Conditions | Yield |
|---|---|
| platinum In neat (no solvent) at 600 - 700°C; acceleration by H2O vapor;; | |
| nickel In neat (no solvent) at 600 - 700°C; acceleration by H2O vapor;; | |
| iron In neat (no solvent) at 600 - 700°C; acceleration by H2O vapor;; |


| Conditions | Yield |
|---|---|
| With magnesium In neat (no solvent) calcination of a mixture of CaO and powdered Mg in an atmosphere of H2;; | |
| With aluminium In neat (no solvent) byproducts: Al2O3; | |
| With magnesium In neat (no solvent) byproducts: MgO; heating mixture at 400°C in vacuum and react. takes place at 550 - 650°C;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) react. was carried out at hydrogen pressure around 50 bar at temp. between 170 and 200°C; |

-
-
7727-37-9
nitrogen

-
-
75-20-7
calcium carbide

-
A
-
7789-78-8
calcium hydride

-
C
-
156-62-7
calcium cyanamide

-
D
-
7440-44-0
pyrographite

| Conditions | Yield |
|---|---|
| In neat (no solvent) |


| Conditions | Yield |
|---|---|
| With hydrogen In neat (no solvent) formation at approx. 500-1000°C;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) smelting together metallic Ca and CaCl2 in vac., in H2- or CH4-stream or in Ar-atmosphere forms no CaCl; CaO and CaH2 are formed by reaction of Ca with H2O;; | A 0% B n/a C n/a |
| In neat (no solvent) smelting together metallic Ca and CaCl2 in vac., in H2- or CH4-stream or in Ar-atmosphere forms no CaCl; CaO and CaH2 are formed by reaction of Ca with H2O;; | A 0% B n/a C n/a |

-
-
7440-70-2
calcium

-
-
74-86-2
acetylene

-
A
-
7789-78-8
calcium hydride

-
B
-
75-20-7
calcium carbide

-
C
-
7440-44-0
pyrographite

| Conditions | Yield |
|---|---|
| In neat (no solvent) dark red heat; crust of CaC2, C and CaH2 inhibit reaction;; | |
| In neat (no solvent) dark red heat; crust of CaC2, C and CaH2 inhibit reaction;; |

-
-
34557-54-5
methane

-
-
7440-70-2
calcium

-
A
-
7789-78-8
calcium hydride

-
B
-
75-20-7
calcium carbide

-
C
-
7440-44-0
pyrographite

| Conditions | Yield |
|---|---|
| In neat (no solvent) dark red heat; crust of CaC2, C and CaH2 inhibit reaction;; | |
| In neat (no solvent) dark red heat; crust of CaC2, C and CaH2 inhibit reaction;; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) Ca form in a NH3-stream at red a mixture of Ca3N2 and CaH2;; | |
| In neat (no solvent) Ca form in a NH3-stream at red a mixture of Ca3N2 and CaH2;; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) heated up to 773 K; XRD; |

| Conditions | Yield |
|---|---|
| With pyrographite In neat (no solvent) at 800 - 900°C; acceleration C; presence of Zn;; | |
| With pyrographite In neat (no solvent) at 1200°C; acceleration by C; in presence of NH3;; | |
| With pyrographite In neat (no solvent) at 900°C; acceleration by C; presence of NaCl;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) Kinetics; decomposition of Ca(NH3)6 to active Ca; different pressures and temps.;; | |
| In neat (no solvent) Kinetics; decomposition of Ca(NH3)6 to active Ca; different pressures and temps.;; |

| Conditions | Yield |
|---|---|
| In not given react. with Ca-salts of phenols at ambient temp. and 200 at;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) ball milled at room temp. for 20 h under 1 MPa of H2; XRD, IR; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) Electric Arc; | |
| In neat (no solvent) Electric Arc; |

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) High Pressure; react. was carried out at hydrogen pressure around 50 bar at temp. between 170 and 200°C; |


-
-
7789-78-8
calcium hydride

| Conditions | Yield |
|---|---|
| In ethanol draining of CaCl2*6H2O at 10°C with alcohol under formation of aq. alcohol-solution with CaH2;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) ball milled at room temp., 120 min; extd. (ethylenediamine) under Ar; filtered; ethylenediamine evapd. at room temp. under 0.05 MPa; XRD; | A 100% B n/a |


-
-
16853-85-3
lithium aluminium tetrahydride

-
-
7789-78-8
calcium hydride

| Conditions | Yield |
|---|---|
| With (C2H5)2O In diethyl ether a mixt. of AlH3, CaH2 and LiAlH4 in Et2O is stirred at 34°C for 5.0 h; flakes are sepd. from soln. and unreacted CaH2 is decanted, flakes are washed with ether and dried in vac. at room temp.; | 100% |
| In tetrahydrofuran a mixt. of AlH3, CaH2 and LiAlH4 in THF is stirred at 30°C for 5.0 h; flakes are sepd. from soln. and unreacted CaH2 is decanted, flakes are washed with ether and dried in vac. at room temp.; | 0% |


| Conditions | Yield |
|---|---|
| With LiAlH4 In diethyl ether; toluene a mixt. of AlH3, CaH2 and LiAlH4 in ether/toluene (1:1) is stirred at 52°C for 3.0 h; flakes are sepd. from soln. and unreacted CaH2 is decanted, flakes are dried in vac. at room temp.; | 100% |
| With LiBH4 In diethyl ether; toluene a mixt. of AlH3, CaH2 and LiBH4 in ether/toluene (1:1) is stirred at 50°C for 2.0 h; flakes are sepd. from soln. and unreacted CaH2 is decanted, flakes are dried in vac. at room temp.; | 100% |
| With LiBH4 In diethyl ether; toluene a mixt. of AlH3, CaH2 and LiBH4 in ether/toluene (1:1) is stirred at 52°C for 5.0 h; flakes are sepd. from soln. and unreacted CaH2 is decanted, flakes are dried in vac. at room temp.; | 100% |

-
-
16853-85-3
lithium aluminium tetrahydride

-
-
7789-78-8
calcium hydride

| Conditions | Yield |
|---|---|
| With (C2H5)2O In diethyl ether a mixt. of AlH3, CaH2 and LiAlH4 in Et2O is stirred at 34°C for 3.0 h; flakes are sepd. from soln. and unreacted CaH2 is decanted, flakes are washed with ether and dried in vac. at room temp., elem. anal.; | 100% |
| With (C2H5)2O In diethyl ether a mixt. of AlH3, CaH2 and LiAlH4 in Et2O is stirred at 34°C for 5.0 h; flakes are sepd. from soln. and unreacted CaH2 is decanted, flakes are washed with ether and dried in vac. at room temp., elem. anal.; | 100% |
| With (C2H5)2O In diethyl ether a mixt. of AlH3, CaH2 and LiAlH4 in Et2O is stirred at 25°C for 7.0 h; flakes are sepd. from soln. and unreacted CaH2 is decanted, flakes are washed with ether and dried in vac. at room temp., elem. anal.; | 95% |
| With (C2H5)2O In diethyl ether a mixt. of AlH3, CaH2 and LiAlH4 in Et2O is stirred at 33°C for 5.0 h; flakes are sepd. from soln. and unreacted CaH2 is decanted, flakes are washed with ether and dried in vac. at room temp., elem. anal.; | 70% |


| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) placing of CaH2 and Ge in welded Ta containers, heating within evacuated, well-flamed, sealed silica jacket at 1150°C for 6 h, cooling to650°C with rate of 10-12°C/h; | 100% |
-
-
7789-78-8
calcium hydride

-
-
359641-96-6
(2-((2,6-diisopropylphenyl)amino)-4-((2,6-diisopropylphenyl)imino)pent-2-ene)ZnCl

-
-
1174669-67-0
({2,6-iPr2H3C6N(CH3)C}2CH)ZnH

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: CaCl2; 0.5 CaH2; | 99% |

| Conditions | Yield |
|---|---|
| With aluminium at 75℃; for 15h; Catalytic behavior; Kinetics; Concentration; Temperature; Milling; | 97.8% |
-
-
109-99-9
tetrahydrofuran

-
-
10043-11-5
ammonia borane complex

-
-
7789-78-8
calcium hydride

-
B
-
1333-74-0
hydrogen

| Conditions | Yield |
|---|---|
| In tetrahydrofuran soln. NH3BH3 in THF was added to suspn. CaH2 in THF, mixt. was stirred overnight, solvent was removed in vac. at room temp. overnight; | A 96% B n/a |


| Conditions | Yield |
|---|---|
| In neat (no solvent, solid phase) placing of CaH2 and Si in welded Ta containers, heating within evacuated, well-flamed, sealed silica jacket at 1150°C for 6 h, cooling to650°C with rate of 10-12°C/h; | 95% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran (inert atm.); stirring suspn. of zinc compd. and CaH2 in THF at 0°C for 3 d; filtration, keeping at -30°C for 24 h, isolation of crystals, elem. anal.; | 91% |

| Conditions | Yield |
|---|---|
| With hydrogen In neat (no solvent) in flow reactor at 180°C; the diluent used was H2; | 90% |
| In neat (no solvent) Kinetics; byproducts: CaF2; mech. activated in rotating flow-through reactor; rotation frequency 100-150 rpm, CaF2 heated at temp. of 180-190°C, SiF4+H2 (1/1 v/v) flow through; gaseous products frozen out with liq. N2; detd. by gas-chromy.; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: CaF2; at 200°C; | 90% |
-
-
7789-78-8
calcium hydride

-
-
7783-80-4
tellurium hexafluoride

-
-
14797-71-8
18O-labeled water

-
A
-
114595-23-2
pentafluorotellurate(VI)

-
B
-
543-54-4
pyridinium hydrogen sulfate

| Conditions | Yield |
|---|---|
| With H2SO4 In pyridine byproducts: H2, CaF2; Sonication; O2 and H2O excluded: H2(18)O added to CaH2 in pyridine, H2 evolution, reactn. mixt. stirred and sonicated (3d, room temp.), degassed, frozen (-196 ° C), TeF6 added (vac. transfer), heated and stirred (45 ° C, 12h), caution: TeF6-pressure!; pyridine and TeF6 removed (vac.), {pyH(1+)}{(18)OTeF5} extracted into warm CH2Cl2, CH2Cl2 removed (vac., white residue), (19)F NMR, concd. H2SO4 added, vac. distn., (19)F NMR; | A 88% B n/a |


| Conditions | Yield |
|---|---|
| elem. anal.; | 85% |

-
-
7789-78-8
calcium hydride

| Conditions | Yield |
|---|---|
| With (C2H5)2O In diethyl ether a mixt. of AlH3, CaH2 and LiBH4 in Et2O is stirred at 20°C for 5.0 h; flakes are sepd. from soln. and unreacted CaH2 is decanted, flakes are dried in vac. at room temp.; | 83% |
| With (C2H5)2O In diethyl ether a mixt. of AlH3, CaH2 and LiBH4 in Et2O is stirred at 22°C for 7.0 h; flakes are sepd. from soln. and unreacted CaH2 is decanted, flakes are dried in vac. at room temp.; | 75% |


| Conditions | Yield |
|---|---|
| With hydrogen In neat (no solvent) formation at passing a mixture of H2 and N2 over CaH2 at 730-750°C after 20 hours;; | 82% |
| With H2 In neat (no solvent) formation at passing a mixture of H2 and N2 over CaH2 at 730-750°C after 20 hours;; | 82% |
| In neat (no solvent) formation in N2-stream at 700-800.degree;; | |
| In neat (no solvent) formation in N2-stream at 700-800.degree;; |
-
-
7789-78-8
calcium hydride

-
-
2160-93-2
N-tert-butyldiethanolamine

-
-
7705-08-0
iron(III) chloride

-
-
7647-14-5
sodium chloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran XRD; | 70% |


| Conditions | Yield |
|---|---|
| In further solvent(s) acetylene carbon black reacted with CaCH2 in Ca/Li flux; sealed ampules heated to 1323 K in 2 h and kept at this temp. for 2 h; cooled to 1073 K in 24 h, cooled to 773 K in 108 h, centrifuged; elem. anal.; | A 70% B n/a |

-
-
67-56-1
methanol

-
-
7789-78-8
calcium hydride

-
-
879089-16-4, 155065-88-6
[Cu2(OAc)4(H2O)2]

-
-
144936-89-0
1,1'-(pyridine-2,6-diyl)bis(4,4-dimethylpentane-1,3-dione)

| Conditions | Yield |
|---|---|
| In methanol under N2; tetraketone added to suspn. of CaH2 in MeOH; mixt. stirred at 20°C for 1 h; Cu(II) acetate monohydrate added; mixt. stirred at 20°C for 16 h; suspn. filtered; solvent removed; dried under vac.; crystn. by vapor diffusion of Et2O in MeOH soln.; | 65% |


| Conditions | Yield |
|---|---|
| With catalyst: TiCl3/Pd In neat (no solvent, solid phase) High Pressure; mixt. of CaB6, CaH2 and catalyst TiCl3/Pd ball milled for 30 min, pelletized under Ar in glove box and then placed in autoclave; H2 pressure 350bar; autoclave heated up to 400-440°C, pressure adjusted to 700 bar and kept for 48 h; XRD; | 60% |
| ruthenium(III)chloride In neat (no solvent, solid phase) High Pressure; mixt. of CaB6, CaH2 and catalyst RuCl3 ball milled for 30 min, pelletized under Ar in glove box and then placed in autoclave; H2 pressure 350 bar; autoclave heated up to 400-440°C, pressure adjusted to 700 barand kept for 48 h; XRD; | |
| titanium(III) chloride In neat (no solvent, solid phase) High Pressure; mixt. of CaB6, CaH2 and catalyst TiCl3 ball milled for 30 min, pelletized under Ar in glove box and then placed in autoclave; H2 pressure 350 bar; autoclave heated up to 400-440°C, pressure adjusted to 700 barand kept for 48 h; XRD; | |
| In neat (no solvent, solid phase) High Pressure; mixt. of CaB6 and CaH2 ball milled for 30 min, pelletized under Ar in glove box and then placed in autoclave; H2 pressure 350 bar; autoclave heated up to 400-440°C, pressure adjusted to 700 bar and kept for 48h; no react. without catalyst; XRD; | 0% |

-
-
7789-78-8
calcium hydride

-
-
119455-79-7
{trans-Pt{C=C(CMe3)P(O)(CCCMe3)C=C(CMe3)(H)}(PET3)2(H2O)}{BF4}

-
-
119455-84-4
{trans-Pt{C=C(CMe3)P(OBF3)(CCCMe3)C=C(CMe3)(H)}{PEt3}2(H)}

-
-
119455-86-6
{trans-Pt{C=C(CMe3)P(O)(CCCMe3)C=C(CMe3)(H)}{PEt3}2(H)}

| Conditions | Yield |
|---|---|
| With H2 In dichloromethane large molar excess of powd. CaH2 added to a soln. of Pt complex (N2), stirred (N2, room temp., 2 h), filtered (filtrate collected in flame-dried glass-ware), stream of H2 gas passed through soln. (2.5 h, -78°C); solvent removed (vac.), residue extd. with hexane and Et2O both twice, Et2O exts. evapd. (vac.), solid crystd. from CH2Cl2/hexane (-20°C); elem. anal. (*1H2O); BF3-free compd. isolated by concn. of the hexane exts. and cooling (-20°C); | A 55% B 18% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) (Ar), mixed with Li/Ca/Ge/CaH2 molar ratio 10:10:2:3 in steal crucible,sealed in silica tube, heated to1323 K in 2 h, kept for 2 h, cooled to 1073 K in 24 h, cooled to 773 K in 108 h; centrifuged, oped (under Ar), XRD; | A 50% B n/a |

-
-
7789-78-8
calcium hydride

-
-
119455-81-1
{trans-Pt{C=C(CMe3)P(O)(CCCMe3)C=C(CMe3)(H)}{P(n-Prop)3}2(H2O)}{BF4}

-
-
119455-85-5
{trans-Pt{C=C(CMe3)P(OBF3)(CCCMe3)C=C(CMe3)(H)}{P(n-Pr3)}2(H)}

| Conditions | Yield |
|---|---|
| With H2 In dichloromethane large molar excess of powd. CaH2 added to a soln. of Pt complex (N2), stirred (N2, room temp., 2 h), filtered (filtrate collected in flame-dried glass-ware), stream of H2 gas passed through soln. (2.5 h, -78°C); solvent removed (vac.), residue extd. with hexane and Et2O both twice, Et2O ext. evapd. (vac.), solid crystd. from CH2Cl2/hexane (-20°C); elem. anal. (*H2O); | 42% |

| Conditions | Yield |
|---|---|
| With lithium at 1050℃; for 5h; Inert atmosphere; Glovebox; Sealed tube; | 42% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) fast reaction at 800°C;; | |
| In neat (no solvent) fast reaction at 800°C;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: CaO; react. in an autoclave under H2-pressure, 300-600°C, exothermic react. (formation of CaH2 from CaCl2 and H2 during the react. possible), addn. of SiO2 for absorption of CaO;; | |
| In neat (no solvent) byproducts: CaO; react. in an autoclave under H2-pressure, 300-600°C, exothermic react. (formation of CaH2 from CaCl2 and H2 during the react. possible), addn. of SiO2 for absorption of CaO;; |


| Conditions | Yield |
|---|---|
| With hydrogen byproducts: CaO; Ca hydride decompd. to nascent H and Ca, redn. with nascent H, Ca reacts with O2 originating from moisture and CaO formes; opt. circumstance of redn. 470°C; sepn. Cr from CaO with dild. aq. HNO3; |

| Conditions | Yield |
|---|---|
| With mineral oil In tetrahydrofuran; water CaH2 dispersed in mixt. of surfactant, mineral oil, C7H8 and methanol; mixt. of THF and water added at 5°C for 5 h; detd. by TEM and SAXS; |
Calcium hydride Chemical Properties
IUPAC Name: Calcium hydride
Molecular Formula: CaH2
Molecular Weight: 42.093880 g/mol
Melting Point: 190 °C(lit.)
Density: 1.70 g/cm3
Sensitive: Moisture
EINECS: 232-189-2
Appearance: Greyish-white solid
Structure of Calcium hydride (CAS NO.7789-78-8):
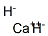
Categories of Calcium hydride (CAS NO.7789-78-8): Inorganics;Adsorbents, Filter Aids and Drying Agents;Other Drying AgentsEssential Chemicals;Materials for Hydrogen Storage;Synthetic Reagents;Metal Hydrides
Calcium hydride Uses
Calcium hydride (CAS NO.7789-78-8) is widely used as a desiccant for basic solvents such as amines and pyridine. It is also used to pre-dry solvents prior to the use of a more reactive desiccant.
Calcium hydride Safety Profile
Hazard Codes:F 
Risk Statements:15
15:Contact with water liberates extremely flammable gases
Safety Statements:24/25-43-7/8-43A
24/25:Avoid contact with skin and eyes
43:In case of fire, use ... (indicate in the space the precise type of fire-fighting equipment. If water increases the risk add - Never use water)
7/8:Keep container tightly closed and dry
43:In case of fire, use ... (indicate in the space the precise type of fire-fighting equipment. If water increases the risk add - Never use water)
Explosive reaction on heating with tetrahydrofuran. Mixtures with potassium chlorate and other metal oxohalogenates (e.g., chlorates; bromates; and perchlorates) are heat- and friction-sensitive explosives. Vigorous or incandescent reaction on heating with halogens (chlorine; bromine; or iodine); manganese dioxide; and silver halides (e.g., silver fluoride; silver iodide). See also CALCIUM COMPOUNDS and HYDRIDES.
Calcium hydride Specification
Calcium hydride (CAS NO.7789-78-8) is hazardous, so the first aid measures and others should be known. Such as: When on the skin: first, should flush skin with plenty of water immediately for at least 15 minutes while removing contaminated clothing. Secondly, get medical aid. Or in the eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Then get medical aid soon. While, it's inhaled: Remove from exposure and move to fresh air immediately. Give artificial respiration while not breathing. When breathing is difficult, give oxygen. And as soon as to get medical aid. Then you have the ingesting of the product: If victim is conscious and alert, give 2-4 cupfuls of milk or water. Get medical aid immediately.
In addition, you should keep it away from sources of ignition. It should be stored in a tightly closed container.
Related Products
- Calcium
- Calcium valproate
- Calcium (1)-bis(2-hydroxy-4-methylvalerate)
- Calcium (2S)-2-[(4-chlorobenzoyl)amino]-3-(1H-indol-3-yl)propanoate
- Calcium (S)-3-methyl-2-oxovalerate
- Calcium 2,4-dichlorophenolate
- Calcium 2-ethylhexanoate
- Calcium 2-hydroxypropanoate
- Calcium 2-methoxyethoxide
- Calcium 2-oxoglutarate
- 7789-79-9
- 7789-80-2
- 7789-82-4
- 7789-92-6
- 7790-21-8
- 779-02-2
- 7790-22-9
- 7790-28-5
- 7790-30-9
- 77903-28-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View