-
Name
Coumarin
- EINECS 202-086-7
- CAS No. 91-64-5
- Article Data299
- CAS DataBase
- Density 1.248 g/cm3
- Solubility 1.7 g/L (20 ºC)
- Melting Point 68-73 °C(lit.)
- Formula C9H6O2
- Boiling Point 297.999 °C at 760 mmHg
- Molecular Weight 146.145
- Flash Point 118.268 °C
- Transport Information UN 2811 6.1/PG 3
- Appearance white crystals or crystalline powder
- Safety 36/37-26
- Risk Codes 20/21/22-36/37/38-40-43
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms cis-o-Coumarinic acid lactone;2H-1-Benzopyran-2-one;Benzo-alpha-pyrone;Rattex;Coumarinic anhydride;5,6-Benzo-alpha-pyrone;2-Oxo-1,2-benzopyran;o-Hydroxycinnamic acid lactone;Benzo-a-pyrone;2-Propenoic acid, 3-(2-hydroxyphenyl)-, d-lactone;o-Coumaric acid lactone;2-Propenoic acid, 3-(2-hydroxyphenyl)-, delta-lactone;5,6-Benzo-2-pyrone;1, 2-Benzopyrone;Cinnamic acid, o-hydroxy-, delta-lactone;Coumarine;2H-chromen-2-one;3-(2-Hydroxyphenyl)-2-propenoic delta-lactone;o-Hydroxycinnamic lactone;
- PSA 30.21000
- LogP 1.79300
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium hydroxide; copper(l) chloride In water; acetonitrile at 25℃; for 0.25h; | 100% |
| With di(n-butyl)tin oxide In 1,4-dioxane Heating; | 97% |
| With hydrogenchloride; N-nitrosopiperidine; potassium iodide In dichloromethane; water at 22℃; for 24h; | 83% |
| With hydrogenchloride; sodium nitrite In dichloromethane; water at 45℃; for 22h; | 64% |
| With sodium carbonate; trifluoroacetic anhydride 1) CH2Cl2, r.t., 2 h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| In methyl 3-(2-oxcyclohexyl)propionate | A n/a B 100% |


| Conditions | Yield |
|---|---|
| In ethylene glycol at 200℃; for 12h; | 99% |

-
-
91-64-5
coumarin

| Conditions | Yield |
|---|---|
| With tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride In dichloromethane at 45℃; for 24h; | 99% |
| tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride In dichloromethane at 20℃; | 70% |
| With Grubbs catalyst first generation In toluene at 80℃; for 2h; Inert atmosphere; | |
| With Hoveyda-Grubbs catalyst second generation In chloroform-d1 at 25℃; for 24h; Reagent/catalyst; Time; Inert atmosphere; Sealed tube; Darkness; | 99 %Spectr. |
-
-
108530-10-5
2-oxo-2H-benzopyran-7-yl trifluoromethanesulfonate

-
-
91-64-5
coumarin

| Conditions | Yield |
|---|---|
| With methanol; zinc; Ni(Ph3P); 1,3-bis-(diphenylphosphino)propane; potassium iodide; zinc In N,N-dimethyl-formamide at 50℃; for 4h; | 98% |
| With 1,1'-bis-(diphenylphosphino)ferrocene; formic acid; palladium diacetate; triethylamine In N,N-dimethyl-formamide at 60℃; for 2h; | 82% |
-
-
20883-98-1
methyl 2-coumarate

-
-
91-64-5
coumarin

| Conditions | Yield |
|---|---|
| In methanol at 40 - 50℃; for 14h; UV-irradiation; | 96% |
| at 750℃; |
-
-
5248-12-4
(α6aH,β6bH,β12bH,α12cH)-cyclobuta<1,2-c,4,3-c1>bis<1>benzopyran-6,7-dione

-
-
91-64-5
coumarin

| Conditions | Yield |
|---|---|
| With 2,4,6-triphenylpyrylium tetrafluoroborate In acetonitrile at 25℃; for 0.333333h; Quantum yield; Mechanism; Irradiation; | 96% |
| With 5-ethyl-1,3-dimethyl-8-(trifluoromethyl)alloxazinium perchlorate In acetonitrile at 20℃; for 0.5h; Catalytic behavior; Reagent/catalyst; Time; Irradiation; Inert atmosphere; Schlenk technique; | 92% |
| With trifluorormethanesulfonic acid; riboflavine tetraacetate In acetonitrile at 20℃; for 0.166667h; Reagent/catalyst; Solvent; Irradiation; Schlenk technique; Inert atmosphere; | 90% |
| In 1,4-dioxane for 0.266667h; Product distribution; Ambient temperature; Irradiation; correlation between photochemical fission and chemical structure; | 25 % Chromat. |
| UV-irradiation; |
-
-
33877-05-3
ethyl 3-(2-methoxyphenyl)acrylate

-
-
91-64-5
coumarin

| Conditions | Yield |
|---|---|
| With boron tribromide In dichloromethane at 50℃; for 5h; Inert atmosphere; | 96% |

-
-
91-64-5
coumarin

| Conditions | Yield |
|---|---|
| With diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; sodium carbonate In dimethyl sulfoxide at 20℃; for 1.5h; Inert atmosphere; Irradiation; | 96% |

| Conditions | Yield |
|---|---|
| With dimethyl cis-but-2-ene-1,4-dioate at 200℃; | 95% |
| Stage #1: C6H4(CH2CH2C(O)O) With di-n-butylboryl trifluoromethanesulfonate; N-ethyl-N,N-diisopropylamine In fluorobenzene; dichloromethane Stage #2: With dichloro( 1,5-cyclooctadiene)platinum(ll); diallylcarbonate; silver trifluoroacetate In fluorobenzene; dichloromethane at 20℃; for 24h; Sealed tube; chemoselective reaction; | 67% |
| With N,N’-di-tert-butylthiadiaziridine-1,1-dioxide; triphenylphosphine; copper(l) chloride at 65℃; for 20h; Inert atmosphere; | 60% |

| Conditions | Yield |
|---|---|
| With acetic acid In methanol for 2h; Ambient temperature; Irradiation; other N-subsituted amides of o-hydroxy-trans-cinnamic acid; | A 95% B 100 % Spectr. |

| Conditions | Yield |
|---|---|
| With pyridine for 5h; Heating; | 95% |
| With acetic acid; silver carbonate In 2,4-dichlorophenoxyacetic acid dimethylamine at 100℃; for 16h; | 63% |
| With copper(l) iodide; triethylamine In dimethyl sulfoxide at 120℃; under 760.051 Torr; for 20h; Inert atmosphere; Schlenk technique; | 57% |
| With bromobenzene; silver carbonate; palladium dichloride In dimethyl sulfoxide at 120℃; for 5h; | |
| With dipotassium peroxodisulfate; silver(I) trifluoromethanethiolate; potassium carbonate In water; dimethyl sulfoxide at 110℃; for 24h; Solvent; Inert atmosphere; Sealed tube; | 33 %Spectr. |
-
-
3943-94-0, 17041-46-2, 6236-62-0
(E)-ethyl 2-hydroxycinnamate

-
-
91-64-5
coumarin

| Conditions | Yield |
|---|---|
| With tris[2-phenylpyridinato-C2,N]iridium(III) In acetonitrile at 20℃; for 24h; Sealed tube; Inert atmosphere; Irradiation; | 94% |
| With tributylphosphine In methanol at 70℃; for 20h; Solvent; Reagent/catalyst; Inert atmosphere; | 82% |
| With palladium on activated charcoal In dimethyl amine at 140℃; Sealed tube; Inert atmosphere; | 78% |
| In benzene for 1h; Irradiation; | 95 % Turnov. |
| With toluene-4-sulfonic acid In poly(methyl methacrylate) for 0.075h; Quantum yield; Reagent/catalyst; UV-irradiation; Darkness; |

| Conditions | Yield |
|---|---|
| With ytterbium(III) trifluoromethanesulfonate hydrate at 80℃; for 0.0333333h; Reagent/catalyst; Microwave irradiation; | 93% |
| With trifluoroacetic acid In chlorobenzene at 100℃; for 1h; Inert atmosphere; | 76% |
| With trifluorormethanesulfonic acid In chlorobenzene at 100℃; for 2h; | 76% |
-
-
151597-71-6
Acrylic acid ((E)-2-propenyl)-phenyl ester

-
-
91-64-5
coumarin

| Conditions | Yield |
|---|---|
| Stage #1: Acrylic acid ((E)-2-propenyl)-phenyl ester With titanium(IV) isopropylate In dichloromethane for 1h; Heating; Stage #2: tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride In dichloromethane for 4h; ring closing metathesis; Heating; | 93% |
-
-
60998-71-2
phenyl prop-2-ynoate

-
-
91-64-5
coumarin

| Conditions | Yield |
|---|---|
| With Echavarren's catalyst In dichloromethane at 18℃; for 1h; | 93% |
| With gold(III) chloride; silver trifluoromethanesulfonate In 1,2-dichloro-ethane at 50℃; | 84% |

-
-
91-64-5
coumarin

| Conditions | Yield |
|---|---|
| With pyridine N-oxide In dichloromethane at 23℃; for 3h; | 93% |

| Conditions | Yield |
|---|---|
| With triethylamine; benzylamine In chloroform at 55℃; for 6h; Reagent/catalyst; Solvent; | 93% |
-
-
17831-88-8
4-chloro-2H-1-benzopyran-2-one

-
A
-
91-64-5
coumarin

-
B
-
118545-81-6
4,4'-bis-(2H-chromen-2-one)

| Conditions | Yield |
|---|---|
| With bis[2-(diphenylphosphino)phenyl] ether; potassium iodide; zinc; bis(triphenylphosphine)nickel(II) chloride In 1,4-dioxane at 130℃; for 0.5h; Ullmann-type reaction; microwave irradiation; | A n/a B 92% |

-
-
91-64-5
coumarin

| Conditions | Yield |
|---|---|
| With tris(2,2'-bipyridyl)ruthenium dichloride; 1,5-dimethoxynaphthalene; ascorbic acid In methanol; water for 5h; Inert atmosphere; Irradiation; | 92% |

| Conditions | Yield |
|---|---|
| With triethylamine; benzylamine In chloroform at 55℃; for 4h; Reagent/catalyst; Solvent; | 92% |

| Conditions | Yield |
|---|---|
| With triethylamine; benzylamine In chloroform at 55℃; for 6h; Reagent/catalyst; Solvent; | 92% |

| Conditions | Yield |
|---|---|
| With polyphosphoric acid In N,N-dimethyl-formamide at 145℃; for 3h; Temperature; Solvent; Concentration; Time; Inert atmosphere; | 91% |
| With polyphosphoric acid In N,N-dimethyl-formamide at 145℃; for 3h; Temperature; Inert atmosphere; | 91% |
| With cesium acetate at 150 - 160℃; for 8h; | 79% |
-
-
90-02-8
salicylaldehyde

-
-
1099-45-2
ethyl (triphenylphosphoranylidene)acetate

-
A
-
3943-94-0, 17041-46-2, 6236-62-0
(E)-ethyl 2-hydroxycinnamate

-
B
-
91-64-5
coumarin

| Conditions | Yield |
|---|---|
| In toluene Heating; | A 91% B 4% |
| In xylene for 4h; Heating; | A 83% B 10% |
| for 4h; Heating; | A 82.8% B 9.6% |

-
-
77924-22-2
7-(1-Phenyl-1H-tetrazol-5-yloxy)-chromen-2-one

-
A
-
5097-82-5
1-phenyl-5-hydroxytetrazole

-
B
-
91-64-5
coumarin

-
C
-
119-84-6
C6H4(CH2CH2C(O)O)

| Conditions | Yield |
|---|---|
| With sodium hypophosphite; palladium on activated charcoal In ethanol; water; benzene for 0.916667h; Heating; | A n/a B 91% C n/a |
| With sodium hypophosphite; palladium on activated charcoal In ethanol; benzene for 55h; Mechanism; Heating; | A n/a B 91% C n/a |

-
-
42974-18-5
3,4-dibromo-3,4-dihydrocoumarin

-
-
91-64-5
coumarin

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; nickel dichloride In methanol at 20℃; for 0.25h; | 90% |
| With sodium sulfide; Aliquat 336 In water; benzene for 1h; Ambient temperature; | 88% |
| With N,N,N,N,-tetramethylethylenediamine; sexithiophene In N,N-dimethyl-formamide for 2h; Inert atmosphere; Irradiation; | 88% |
| In methanol at 20℃; for 6h; Irradiation; | 65% |

| Conditions | Yield |
|---|---|
| palladium-carbon | 90% |

| Conditions | Yield |
|---|---|
| With N,N-diethylaniline at 210 - 215℃; for 4h; | 89.2% |
| With N,N-diethylaniline In xylene for 4h; Heating; | 89% |

| Conditions | Yield |
|---|---|
| With tetraphosphorus decasulfide In 5,5-dimethyl-1,3-cyclohexadiene for 12h; Inert atmosphere; Reflux; | 100% |
| With Lawessons reagent for 0.05h; microwave irradiation; | 98% |
| With bis(tricyclohexyltin)sulfide; boron trichloride for 7h; Heating; | 94% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid | 100% |
| Stage #1: coumarin With sulfuric acid at 4℃; for 0.166667h; Stage #2: With guanidine nitrate | 98% |
| With ammonium cerium(IV) nitrate; acetic acid at 20℃; for 2h; | 92% |

| Conditions | Yield |
|---|---|
| With formic acid; palladium In methanol; water Ambient temperature; | 100% |
| With hydrogen; W-2 Raney nickel In ethyl acetate for 0.833333h; Ambient temperature; Irradiation; | 100% |
| With hydrogen; palladium on activated charcoal In acetic acid under 760.051 Torr; for 12h; | 99% |

-
-
91-64-5
coumarin

| Conditions | Yield |
|---|---|
| In hexane ligand was added to hexane soln. of Os-cluster in Schlenk tube, stirred for 100 min at room temp. under N2; solvent was removed under reduced pressure, recrystd. from CH2Cl2/hexaneat -5°C; elem. anal.; | 99% |
-
-
91-64-5
coumarin

-
-
116295-83-1
coumarin-d6

| Conditions | Yield |
|---|---|
| With 10% Pt/activated carbon; water-d2 In cyclohexane; isopropyl alcohol at 80℃; for 24h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-ethoxy-1-(tert-butyldimethylsilyloxy)ethene; coumarin With C18H14F18O15S6 In dichloromethane at -78℃; for 0.5h; Inert atmosphere; Stage #2: cyclohexanone In dichloromethane at -78℃; for 0.5h; Reagent/catalyst; Inert atmosphere; | 99% |


| Conditions | Yield |
|---|---|
| With ytterbium(III) trifluoromethanesulfonate nonohydrate In neat (no solvent) for 0.0833333h; Milling; Microwave irradiation; | 98% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In water at 80℃; for 16h; Solvent; Temperature; Reagent/catalyst; stereoselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| In toluene at 25℃; for 0.583333h; Concentration; Solvent; Time; Inert atmosphere; UV-irradiation; Flow reactor; diastereoselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| With trimethylsilylazide; bis-[(trifluoroacetoxy)iodo]benzene In dichloromethane at 20℃; for 0.5h; Solvent; Reagent/catalyst; | 98% |
| With bis-[(trifluoroacetoxy)iodo]benzene In dichloromethane at 20℃; for 1h; Reagent/catalyst; Solvent; regioselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| With bis-[(trifluoroacetoxy)iodo]benzene In dichloromethane at 20℃; for 1.5h; regioselective reaction; | 98% |
-
-
63930-16-5
1,2-bis(3-fluorophenyl)diselane

-
-
91-64-5
coumarin

| Conditions | Yield |
|---|---|
| With bis-[(trifluoroacetoxy)iodo]benzene In dichloromethane at 20℃; for 2h; regioselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| With bis-[(trifluoroacetoxy)iodo]benzene In dichloromethane at 20℃; for 1h; regioselective reaction; | 98% |
-
-
563-79-1
2,3-Dimethyl-2-butene

-
-
91-64-5
coumarin

-
-
7305-18-2
1,1,2,2-tetramethyl-2,2adihydro-1H-cyclobuta[c]chromen-3(8bH)-one

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide In dichloromethane; 1,2-dibromomethane at 20℃; for 5h; Inert atmosphere; Irradiation; | 97% |

| Conditions | Yield |
|---|---|
| With 5-Nitro-1,10-phenanthroline; oxygen; palladium diacetate In N,N-dimethyl-formamide at 80℃; for 24h; Heck reaction; chemoselective reaction; | 97% |
| With 1,10-Phenanthroline; oxygen In N,N-dimethyl-formamide at 100℃; for 24h; Catalytic behavior; Solvent; Suzuki Coupling; Sealed tube; regioselective reaction; | 87% |
| With 1,10-Phenanthroline; oxygen; palladium diacetate In N,N-dimethyl-formamide at 100℃; for 24h; Sealed tube; | 82% |
| With 5-Nitro-1,10-phenanthroline; oxygen; palladium diacetate In N,N-dimethyl-formamide at 80℃; for 12h; Schlenk technique; Sealed tube; | 60% |
| Stage #1: coumarin With 1,10-Phenanthroline; oxygen; palladium diacetate In acetonitrile for 0.0833333h; Heck type reaction; Stage #2: phenylboronic acid In N,N-dimethyl-formamide at 100℃; Heck type reaction; regioselective reaction; | 14% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-ethoxy-1-(tert-butyldimethylsilyloxy)ethene; coumarin With C18H14F18O15S6 In dichloromethane at -78℃; for 0.5h; Inert atmosphere; Stage #2: acetone In dichloromethane at -78℃; for 0.5h; Inert atmosphere; | 97% |


| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; di-tert-butyl peroxide at 120℃; for 6h; | 97% |
| With di-tert-butyl peroxide; potassium iodide at 120℃; for 20h; Sealed tube; | 77% |

| Conditions | Yield |
|---|---|
| With (BQ‑NCOP)IrHCl; sodium t-butanolate at 60℃; for 8h; Inert atmosphere; Schlenk technique; Sealed tube; chemoselective reaction; | 97% |
-
-
91806-16-5
1,9-dimethyl-3,4-dihydro-β-carboline

-
-
91-64-5
coumarin

| Conditions | Yield |
|---|---|
| With sodium carbonate In water at 80℃; for 20h; stereoselective reaction; | 97% |

| Conditions | Yield |
|---|---|
| With iron(III) chloride; sulfur In ethanol at 60℃; for 2h; Sealed tube; | 97% |
-
-
20541-48-4
1,2-bis(4-bromophenyl)diselenide

-
-
91-64-5
coumarin

| Conditions | Yield |
|---|---|
| With bis-[(trifluoroacetoxy)iodo]benzene In dichloromethane at 20℃; for 1.5h; | 97% |
| With bis-[(trifluoroacetoxy)iodo]benzene In dichloromethane at 20℃; for 1.5h; regioselective reaction; | 97% |
Coumarin Consensus Reports
Coumarin Specification
The CAS registry number of Coumarin is 91-64-5. The systematic name is 2H-chromen-2-one. In addition, it is a fragrant chemical compound of benzopyrone found in many plants with the molecular formula C9H6O2. What's more, it has a sweet odor, and readily recognised as the scent of newly-mown hay. So it can be used in perfumes.
Physical properties about this chemical are: (1)ACD/LogP: 1.39; (2)ACD/LogD (pH 5.5): 1.39; (3)ACD/LogD (pH 7.4): 1.39; (4)ACD/BCF (pH 5.5): 6.705; (5)ACD/BCF (pH 7.4): 6.705; (6)ACD/KOC (pH 5.5): 135.881; (7)ACD/KOC (pH 7.4): 135.881; (8)#H bond acceptors: 2; (9)Polar Surface Area: 26.3 Å2; (10)Index of Refraction: 1.595; (11)Molar Refractivity: 39.767 cm3; (12)Molar Volume: 117.097 cm3; (13)Polarizability: 15.765 ×10-24cm3; (14)Surface Tension: 46.411 dyne/cm; (15)Density: 1.248 g/cm3; (16)Flash Point: 118.268 °C; (17)Enthalpy of Vaporization: 53.789 kJ/mol; (18)Boiling Point: 297.999 °C at 760 mmHg; (19)Vapour Pressure: 0.001 mmHg at 25°C.
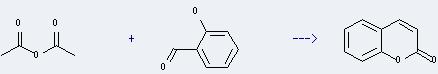
Preparation of Coumarin: it can be prepared in a laboratory in a Perkin reaction between salicylaldehyde and acetic anhydride. The reaction needs solvent potassium carbonate and the temperature should be controlled at 210-212 °C. After the reaction, via a series of washing, vacuum distillation and recrystallization by ethanol you can get the desired product.
Uses of Coumarin: It has been used as aroma enhancer in pipe tobaccos and certain alcoholic drinks. And it is used in the pharmaceutical industry as a precursor molecule in the synthesis of a number of synthetic anticoagulant pharmaceuticals similar to dicoumarol. In addition, it is also used as a gain medium in some dye lasers, and as a sensitizer in older photovoltaic technologies. Moreover, it can be used to get chromene-2-thione. This reaction will need reagent bis(1,5-cyclooctanediylboryl) sulfide and solvent heptane. The reaction time is 2 hours by heating. The yield is about 80%.
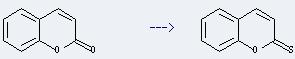
When you are using this chemical, please be cautious about it as the following:
This chemical is harmful by inhalation, in contact with skin and if swallowed. It is irritating to eyes, respiratory system and skin. In addition, it may cause sensitization by skin contact and is moderately toxic to the liver and kidneys. When you are using it, wear suitable protective clothing and gloves. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: c1ccc2c(c1)ccc(=O)o2
(2)Std. InChI: InChI=1S/C9H6O2/c10-9-6-5-7-3-1-2-4-8(7)11-9/h1-6H
(3)Std. InChIKey: ZYGHJZDHTFUPRJ-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | oral | 202mg/kg (202mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LIVER: OTHER CHANGES | Food and Cosmetics Toxicology. Vol. 2, Pg. 327, 1964. |
| man | TDLo | oral | 87mg/kg/17W-I (87mg/kg) | LIVER: LIVER FUNCTION TESTS IMPAIRED | Human Toxicology. Vol. 8, Pg. 501, 1989. |
| mouse | LD50 | intraperitoneal | 220mg/kg (220mg/kg) | BEHAVIORAL: ANALGESIA | Arzneimittel-Forschung. Drug Research. Vol. 15, Pg. 897, 1965. |
| mouse | LD50 | oral | 196mg/kg (196mg/kg) | Yakugaku Zasshi. Journal of Pharmacy. Vol. 83, Pg. 1124, 1963. | |
| mouse | LD50 | subcutaneous | 242mg/kg (242mg/kg) | Yakugaku Zasshi. Journal of Pharmacy. Vol. 83, Pg. 1124, 1963. | |
| rat | LD50 | oral | 293mg/kg (293mg/kg) | Food and Cosmetics Toxicology. Vol. 12, Pg. 385, 1974. | |
| women | TDLo | oral | 30mg/kg/30D-I (30mg/kg) | LIVER: LIVER FUNCTION TESTS IMPAIRED | Human Toxicology. Vol. 8, Pg. 501, 1989. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View