-
Name
Cyproheptadine
- EINECS 204-928-9
- CAS No. 129-03-3
- Article Data10
- CAS DataBase
- Density 1.115g/cm3
- Solubility 317.6ug/L(22.5 oC)
- Melting Point 112.3-113.3°
- Formula C21H21 N
- Boiling Point 440.1°Cat760mmHg
- Molecular Weight 287.404
- Flash Point 194.5°C
- Transport Information
- Appearance Colorless liquid, sweet, almond odor.
- Safety Poison by ingestion, intraperitoneal, subcutaneous and intravenous routes. Experimental teratogenic and reproductive effects. When heated to decomposition it emits toxic fumes of NOx.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 1-Methyl-4-(5H-dibenzo[a,d]cycloheptenylidene)piperidine;1-Methyl-4-(dibenzo[a,e]cycloheptatrien-5-ylidene)piperidine;4-(5H-Dibenzo[a,d]cyclohepten-5-ylidene)-1-methylpiperidine; Cycloheptadine;Cyproheptadine; Periactinol
- PSA 3.24000
- LogP 5.43780
Synthetic route

| Conditions | Yield |
|---|---|
| With formic acid at 100℃; for 2h; | 99% |
| With hydrogenchloride; acetic acid at 120℃; for 2h; | 83% |
| With formic acid for 1h; Heating; Yield given; |
-
-
121138-82-7
ethyl 4-(5H-dibenzocyclohepten-5-ylidene)-1-piperidinecarboxylate

-
-
129-03-3
cyproheptadine

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran for 2h; Heating; Yield given; |
-
-
63463-36-5
(1-methyl-4-piperidyl)magnesium chloride

-
-
129-03-3
cyproheptadine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 76 percent / tetrahydrofuran / 70 °C 2: 83 percent / HOAc, conc. HCl / 2 h / 120 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 76 percent / tetrahydrofuran / 70 °C 2: 83 percent / HOAc, conc. HCl / 2 h / 120 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 60 percent / TiCl3 / 1,2-dimethoxy-ethane 2: LiAlH4 / tetrahydrofuran / 2 h / Heating View Scheme |
-
-
29976-53-2
N-ethoxycarbonyl-4-piperidone

-
-
129-03-3
cyproheptadine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / TiCl3 / 1,2-dimethoxy-ethane 2: LiAlH4 / tetrahydrofuran / 2 h / Heating View Scheme |
-
-
129-03-3
cyproheptadine

-
-
541-41-3
chloroformic acid ethyl ester

-
-
121138-82-7
ethyl 4-(5H-dibenzocyclohepten-5-ylidene)-1-piperidinecarboxylate

| Conditions | Yield |
|---|---|
| In toluene for 5h; Heating; | 94% |
| In hexane; toluene | |
| In toluene for 3h; Reflux; |
-
-
129-03-3
cyproheptadine

-
-
21081-07-2
4-(10,11-dihydro-5H-dibenzocyclohepten-5-ylidene)-1-methylpiperidine

| Conditions | Yield |
|---|---|
| With hydrogen In ethanol; acetic acid | 94% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 48h; regioselective reaction; | 72% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 48h; regioselective reaction; | 63% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 48h; regioselective reaction; | 55% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 4h; regioselective reaction; | 51% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 4h; regioselective reaction; | 48% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 48h; regioselective reaction; | 47% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 16h; regioselective reaction; | 45% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 48h; regioselective reaction; | 44% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 48h; regioselective reaction; | 41% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 48h; regioselective reaction; | 41% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 48h; Catalytic behavior; Reagent/catalyst; Solvent; Time; regioselective reaction; | 41% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 4h; regioselective reaction; | 38% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 16h; regioselective reaction; | 38% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 16h; regioselective reaction; | 38% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 4h; regioselective reaction; | 34% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 48h; regioselective reaction; | 34% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 4h; regioselective reaction; | 32% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 16h; regioselective reaction; | 31% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 16h; regioselective reaction; | 19% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 48h; regioselective reaction; | 15% |

| Conditions | Yield |
|---|---|
| With PdCl(1,4-bis(diphenylphosphino)butane)(C3H5); potassium acetate In N,N-dimethyl acetamide at 150℃; for 16h; regioselective reaction; | A n/a B 11% |

-
-
129-03-3
cyproheptadine

-
-
54191-04-7
cyproheptadine 10,11-epoxide

| Conditions | Yield |
|---|---|
| With sodium hydroxide; dihydrogen peroxide In acetone; acetonitrile |
-
-
129-03-3
cyproheptadine

-
-
50893-53-3
carbonochloridic acid 1-chloro-ethyl ester

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,2-dichloro-ethane 1) 0 deg C, 2) reflux; |
-
-
129-03-3
cyproheptadine

-
-
14051-46-8
4-(5H-dibenzo[a,d]cyclohepten-5-ylidene)piperidine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Na2CO3 / 1,2-dichloro-ethane / 1) 0 deg C, 2) reflux 2: 1) CH3OH, 2) NaOH / 1) r.t., 15 min, 50 deg C, 15 min, 2) water View Scheme | |
| Multi-step reaction with 2 steps 1: toluene / 3 h / Reflux 2: potassium hydroxide; water / ethanol / Reflux View Scheme |
-
-
129-03-3
cyproheptadine

-
-
175692-31-6
4-(4-Dibenzo[a,d]cyclohepten-5-ylidene-piperidin-1-yl)-butylamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: Na2CO3 / 1,2-dichloro-ethane / 1) 0 deg C, 2) reflux 2: 1) CH3OH, 2) NaOH / 1) r.t., 15 min, 50 deg C, 15 min, 2) water 3: 80 percent / Na2CO3, KI / acetonitrile; dimethylformamide / Heating 4: 62 percent / LiAlH4 / tetrahydrofuran; diethyl ether / 2 h / Ambient temperature View Scheme |
Cyproheptadine Chemical Properties
Product Name: Cyproheptadine
CAS: 129-03-3
Formula: C21H21N
Molecular Weight: 287.4
Molecular Structure of Cyproheptadine (CAS NO.129-03-3):
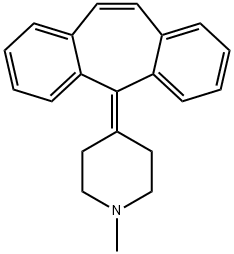
Density: 1.115 g/cm3
Flash Point: 194.5 °C
Boiling Point: 440.1 °C at 760 mmHg
Index of Refraction: 1.629
Molar Refractivity: 91.59 cm3
Molar Volume: 257.5 cm3
Polarizability: 36.31×10-24cm3
Surface Tension: 45.6 dyne/cm
Enthalpy of Vaporization: 69.72 kJ/mol
Vapour Pressure: 6.03E-08 mmHg at 25°C
Water Solubility: 1.135(mg/L) at 25°C
Cyproheptadine Toxicity Data With Reference
| 1. | orl-rat LD50:295 mg/kg | 27ZQAG Psychotropic Drugs and Related Compounds E. Usdin andD.H. Efron,2nd ed.,Washington, DC.: 1972,69. | ||
| 2. | ipr-rat LD50:52 mg/kg | 27ZQAG Psychotropic Drugs and Related Compounds E. Usdin andD.H. Efron,2nd ed.,Washington, DC.: 1972,69. | ||
| 3. | orl-mus LD50:106 mg/kg | ARZNAD Arzneimittel-Forschung. Drug Research. 25 (1975),1723. | ||
| 4. | scu-mus LD50:107 mg/kg | 27ZQAG Psychotropic Drugs and Related Compounds E. Usdin andD.H. Efron,2nd ed.,Washington, DC.: 1972,69. | ||
| 5. | orl-dog LDLo:50 mg/kg | 27ZIAQ Drug Dosages in Laboratory Animals-A Handbook C.D. Barnes andL.G. Eltherington,Berkeley, CA.: Univ. of California Press,1973,82. | ||
| 6. | ivn-rbt LDLo:3500 µg/kg | 27ZQAG Psychotropic Drugs and Related Compounds E. Usdin andD.H. Efron,2nd ed.,Washington, DC.: 1972,69. |
Cyproheptadine Consensus Reports
EPA Genetic Toxicology Program.
Cyproheptadine Safety Profile
Poison by ingestion, intraperitoneal, subcutaneous and intravenous routes. Experimental teratogenic and reproductive effects. When heated to decomposition it emits toxic fumes of NOx.
Safety Information about Cyproheptadine (CAS NO.129-03-3):
HS Code: 29143900
Cyproheptadine Specification
The chemical synonyms of Cyproheptadine (CAS NO.129-03-3) are 1-Methyl-4-(5-dibenzo(a,e)cycloheptatrienylidene)piperidine ; 1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)piperidine ; 4-(5-Dibenzo(a,d)cyclohepten-5-ylidine)-1-methylpiperidine ; 4-(5H-Dibenzo(a,d)cyclohepten-5-ylidene)-1-methylpiperidene ; 4-(5H-Dibenzo(a,d)cyclohepten-5-ylidene)-1-methylpiperidine ; 5-(1-Methylpiperidylidene-4)-5H-dibenzo(a,d)cyclopheptene ; 5-20-08-00500 (Beilstein Handbook Reference) ; BRN 1685976 ; CCRIS 5232 ; Ciproheptadina ; Ciproheptadina [INN-Spanish] ; Cyproheptadine ; Cyproheptadinum ; Cyproheptadinum [INN-Latin] ; Dronactin ; EINECS 204-928-9 ; Eiproheptadine ; HSDB 3048 ; MK 141 ; Periactin ; Periactinol ; UNII-2YHB6175DO .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View