-
Name
D-Plenylglycinol
- EINECS 260-287-5
- CAS No. 56613-80-0
- Article Data150
- CAS DataBase
- Density 1.104 g/cm3
- Solubility Slightly soluble in water.
- Melting Point 76-79 °C
- Formula C8H11NO
- Boiling Point 261 °C at 760 mmHg
- Molecular Weight 137.181
- Flash Point 125.3 °C
- Transport Information
- Appearance white to light yellow crystal powder
- Safety 22-24/25-45-36/37/39-26
- Risk Codes 34-36/37/38-23/24/25
-
Molecular Structure
-
Hazard Symbols
 C,
C,  T
T
- Synonyms Benzeneethanol,b-amino-, (R)-;(-)-(R)-b-Aminobenzeneethanol;(-)-2-Amino-2-phenylethanol;(-)-Phenylglycinol;(2R)-2-Amino-2-phenylethanol;(R)-(-)-2-Amino-2-phenylethanol;(R)-(-)-2-Phenyl-2-aminoethanol;(R)-(-)-2-Phenylglycinol;(R)-(-)-Phenylglycinol;(R)-2-Amino-2-phenylethanol;(R)-2-Phenylglycinol;(R)-Phenylglycinol;(R)-a-Phenylglycinol;(bR)-b-Aminobenzeneethanol;D-(-)-2-Amino-2-phenylethanol;D-(-)-a-Phenylglycinol;D-a-Phenylglycinol;R-(-)-a-Phenylglycinol;[(R)-2-Hydroxy-1-phenylethyl]amine;R(-)-2-Phenylglycinol;D-Phg-ol;
- PSA 46.25000
- LogP 1.37900
Synthetic route
-
-
1175712-18-1, 1175712-19-2
N-[(1R)-2-hydroxy-1-phenylethyl]-N-[(1S)-1-(tributylstannyl)but-3-en-1-yl]benzenesulfonamide

-
A
-
56613-80-0
(R)-Phenylglycinol

-
B
-
813-19-4
bis(tri-n-butyltin)

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydrogensulfate In acetonitrile Inert atmosphere; Electrolysis; | A 96% B n/a |
-
-
875-74-1
(R)-phenylglycine

-
-
56613-80-0
(R)-Phenylglycinol

| Conditions | Yield |
|---|---|
| Stage #1: (R)-phenylglycine With sodium tetrahydroborate; iodine In tetrahydrofuran at 64℃; for 18h; Stage #2: With methanol In tetrahydrofuran at 20℃; | 94% |
| With lithium borohydride; chloro-trimethyl-silane In tetrahydrofuran at 0 - 20℃; for 49h; | 93% |
| With lithium aluminium tetrahydride In tetrahydrofuran for 20h; Heating; | 92% |
-
-
126923-26-0
(R)-2-azido-2-phenylethan-1-ol

-
-
56613-80-0
(R)-Phenylglycinol

| Conditions | Yield |
|---|---|
| With water; triphenylphosphine In tetrahydrofuran at 70℃; Staudinger Azide Reduction; | 92% |
| With hydrogen; palladium on activated charcoal |
-
-
78761-26-9
N-((1R)-2-hydroxy-1-phenylethyl)acetamide

-
-
56613-80-0
(R)-Phenylglycinol

| Conditions | Yield |
|---|---|
| Stage #1: N-[(1R)-2-hydroxy-1-phenylethyl]acetamide With Schwartz's reagent In tetrahydrofuran at 20℃; for 0.0666667h; Inert atmosphere; Stage #2: With water In tetrahydrofuran Inert atmosphere; | 89% |
-
-
87319-84-4
(1'R,3R)-3-Hydroxy-N-(2-hydroxy-1-phenylethyl)-4-methylpentanamid

-
A
-
56613-80-0
(R)-Phenylglycinol

-
B
-
77981-87-4
(R)-3-hydroxy-4-methylpentanoic acid

-
C
-
63674-22-6
(3S)-3-hydroxy-4-methylpentanoic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol; water for 15h; Heating; | A n/a B 88% C n/a |

-
-
87319-85-5
(1'R,3S)-3-Hydroxy-N-(2-hydroxy-1-phenylethyl)-hexanamid

-
A
-
77877-35-1
(-)-(3R)-3-hydroxyhexanoic acid

-
B
-
66997-60-2
(+)-(3S)-3-hydroxyhexanoic acid

-
C
-
56613-80-0
(R)-Phenylglycinol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol; water for 15h; Heating; | A n/a B 86% C n/a |

-
-
39251-40-6
(R)-amino-phenyl-acetic acid ethyl ester

-
-
56613-80-0
(R)-Phenylglycinol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride | |
| With methanol; nickel at 40℃; under 110326 Torr; Hydrogenation; |
-
-
14231-57-3
(R)-N-benzyl-2-phenylglycinol

-
-
56613-80-0
(R)-Phenylglycinol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium dichloride In methanol for 1h; |
-
-
134356-93-7
(1R,2R,5R)-3-[(E)-(R)-2-Hydroxy-1-phenyl-ethylimino]-2,6,6-trimethyl-bicyclo[3.1.1]heptan-2-ol

-
-
56613-80-0
(R)-Phenylglycinol

| Conditions | Yield |
|---|---|
| With hydroxylamine acetate In ethanol for 20h; Ambient temperature; Yield given; |
-
-
4427-92-3, 90971-11-2, 129097-94-5, 90970-80-2
(4S)-4-phenyl[1,3]dioxolan-2-one

-
A
-
56613-81-1
(S)-2-Amino-1-phenyl-1-ethanol

-
B
-
56613-80-0
(R)-Phenylglycinol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium azide; water; hydrogen; palladium on activated charcoal 1.) DMF, 70 deg C, 48 h; 2.) ethanol, r.t.; Yield given. Multistep reaction; |
-
-
2935-35-5
(S)-2-phenylglycine

-
-
56613-80-0
(R)-Phenylglycinol

| Conditions | Yield |
|---|---|
| With lithium borohydride; sulfuric acid In tetrahydrofuran for 24h; Ambient temperature; |
-
-
102089-74-7, 117049-14-6, 138457-46-2, 67341-01-9
tert-butyl 2-hydroxy-1-phenylethylcarbamate

-
A
-
56613-80-0
(R)-Phenylglycinol

-
B
-
20989-17-7
(2S)-2-phenylglycinol

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane for 1h; Ambient temperature; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |

| Conditions | Yield |
|---|---|
| at 40℃; under 110326 Torr; Hydrogenation; |


-
-
56613-80-0
(R)-Phenylglycinol

| Conditions | Yield |
|---|---|
| With ethanol; palladium Hydrogenation; |
-
-
67341-01-9, 117049-14-6, 138457-46-2, 102089-74-7
(R)-N-(tert-butoxycarbonyl)phenylglycinol

-
-
56613-80-0
(R)-Phenylglycinol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran for 48h; BOC-deprotection; |
-
-
342651-76-7
(RS,1R)-2-methylpropane-2-sulfinic acid [1-((tert-butyldimethylsilanyloxy)methyl)-1-phenylethyl]amide

-
-
56613-80-0
(R)-Phenylglycinol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane; methanol at 20℃; for 0.5h; |
-
-
131934-09-3, 135765-81-0, 712-41-4
ethyl 2-(hydroxyimino)-2-phenylacetate

-
A
-
56613-80-0
(R)-Phenylglycinol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 85 percent / NaBH4; I2 / tetrahydrofuran / 4 h / Heating 2.1: dibenzoyl-L-tartaric acid / acetone / 6 h / 25 °C 2.2: aq. KOH / CH2Cl2 View Scheme |
-
-
350479-90-2
(S,E)-N-benzylidene-4-methylbenzenesulfinamide

-
-
56613-80-0
(R)-Phenylglycinol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 98 percent / i-Pr2NH; BuLi / tetrahydrofuran; hexane / 0.25 h / -78 °C 2: 95 percent / TFA / methanol / 15 h / 20 °C 3: 91 percent / Et3N / acetonitrile / 15 h / 20 °C 4: sym-collidine; TFAA / acetonitrile / 0.25 h / 0 °C 5: 1N HCl / tetrahydrofuran / 48 h View Scheme | |
| Multi-step reaction with 5 steps 1: i-Pr2NH; BuLi / tetrahydrofuran; hexane / 0 °C 2: 95 percent / TFA / methanol / 15 h / 20 °C 3: 91 percent / Et3N / acetonitrile / 15 h / 20 °C 4: sym-collidine; TFAA / acetonitrile / 0.25 h / 0 °C 5: 1N HCl / tetrahydrofuran / 48 h View Scheme |
-
-
280128-39-4
(1R,RS)-1-phenyl-2-p-tolylsulfinylethylamine

-
-
56613-80-0
(R)-Phenylglycinol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 91 percent / Et3N / acetonitrile / 15 h / 20 °C 2: sym-collidine; TFAA / acetonitrile / 0.25 h / 0 °C 3: 1N HCl / tetrahydrofuran / 48 h View Scheme |
-
-
280128-43-0
tert-butyl N-[(1R,RS)-1-phenyl-2-p-tolylsulfinylethyl]carbamate

-
-
56613-80-0
(R)-Phenylglycinol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sym-collidine; TFAA / acetonitrile / 0.25 h / 0 °C 2: 1N HCl / tetrahydrofuran / 48 h View Scheme |
-
-
98-01-1
furfural

-
-
56613-80-0
(R)-Phenylglycinol

-
-
139437-47-1
(R)-2-{[1-Furan-2-yl-meth-(E)-ylidene]-amino}-2-phenyl-ethanol

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 20℃; for 12h; | 100% |
| In benzene Heating; | 88% |
| In benzene Heating; | 85% |
| With magnesium sulfate In dichloromethane | |
| In toluene Condensation; Heating; |
-
-
498-62-4
3-thiophene carboxaldehyde

-
-
56613-80-0
(R)-Phenylglycinol

-
-
139437-49-3
(R)-2-Phenyl-2-{[1-thiophen-3-yl-meth-(E)-ylidene]-amino}-ethanol

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 20℃; for 12h; | 100% |
| In benzene Heating; | 92% |
-
-
4079-52-1
methoxymethyl phenyl ketone

-
-
56613-80-0
(R)-Phenylglycinol

-
-
103621-11-0, 103621-12-1
(R)-2,4-diphenyl-2-(methoxymethyl)-1,3-oxazolidine

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 72h; Heating; | 100% |
-
-
13122-67-3
4-oxo-6-phenylhexanoic acid

-
-
56613-80-0
(R)-Phenylglycinol

-
-
675589-40-9
(3R,7aS)-3-phenyl-7a-(2-phenylethyl)-2,3,7,7a-tetrahydropyrrolo[2,1-b]oxazol-5-one

| Conditions | Yield |
|---|---|
| In toluene for 36h; Reflux; | 100% |
| In toluene at 130℃; for 36h; | 99% |
| Heating; | 77% |
| In toluene Heating; | 76.5% |
-
-
56613-80-0
(R)-Phenylglycinol

-
-
100-52-7
benzaldehyde

-
-
153924-63-1
β-[(phenylmethylene)amino]-[R-(E)-]-benzeneethanol

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 20℃; for 12h; | 100% |
| In benzene for 4h; Heating; | 94% |
| In benzene for 3h; Heating; | 94% |
-
-
35086-79-4, 95602-71-4
cinnamoyl chloride

-
-
56613-80-0
(R)-Phenylglycinol

-
-
185761-28-8
(E)-3-(4-Chloro-phenyl)-N-((R)-2-hydroxy-1-phenyl-ethyl)-acrylamide

| Conditions | Yield |
|---|---|
| With sodium carbonate In dichloromethane Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 20℃; for 12h; | 100% |

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 20℃; for 12h; | 100% |
-
-
56613-80-0
(R)-Phenylglycinol

-
-
1018435-02-3
(1,1-difluoroprop-1-en-2-yloxy)trimethylsilane

-
-
1018434-46-2
(E)-2,2-difluoro-1-methylethylidene((1R)-2-trimethylsilyloxy-1-phenylethyl)amine

| Conditions | Yield |
|---|---|
| In diethyl ether | 100% |

| Conditions | Yield |
|---|---|
| In methanol Michael addition; | 100% |
| In methanol for 3h; Reflux; |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 20h; Inert atmosphere; | 100% |
-
-
56613-80-0
(R)-Phenylglycinol

-
-
3320-86-3
2-nitrophenyl isocyanate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 1h; | 100% |
-
-
56613-80-0
(R)-Phenylglycinol

-
-
107-02-8
acrolein

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; | 100% |
| In chloroform at 20℃; for 0.0833333h; |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
56613-80-0
(R)-Phenylglycinol

-
-
67341-01-9, 117049-14-6, 138457-46-2, 102089-74-7
(R)-N-(tert-butoxycarbonyl)phenylglycinol

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; | 99% |
| With triethylamine In tetrahydrofuran at 0℃; for 3h; | 99% |
| With triethylamine In tetrahydrofuran at 0℃; for 3h; Inert atmosphere; | 99% |
-
-
56613-80-0
(R)-Phenylglycinol

-
-
79-04-9
chloroacetyl chloride

-
-
94193-77-8
(R)-2-chloro-N-(2-hydroxy-1-phenylethyl)acetamide

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 25℃; | 99% |
| With sodium hydroxide In dichloromethane; water at 0℃; for 1.5h; | 76% |
| With triethylamine In tetrahydrofuran; ethanol for 10h; | 64% |
| With triethylamine In tetrahydrofuran at 0℃; for 0.166667h; Inert atmosphere; | 3.54 g |
-
-
2426-02-0
3,4,5,6-Tetrahydrophthalic anhydride

-
-
56613-80-0
(R)-Phenylglycinol

-
-
938082-20-3
2-[(1R)-2-hydroxy-1-phenylethyl]-4,5,6,7-tetrahydro-1H-isoindole-1,3(2H)-dione

| Conditions | Yield |
|---|---|
| In toluene for 3h; Heating; | 99% |
| In toluene Heating; | 97% |
-
-
56613-80-0
(R)-Phenylglycinol

-
-
115-80-0
Triethyl orthopropionate

-
-
205178-47-8
(4R)-2-ethyl-4-phenyl-2-oxazoline

| Conditions | Yield |
|---|---|
| With acetic acid In 1,2-dichloro-ethane for 2h; Cyclization; Heating; | 99% |
-
-
6092-54-2
n-hexyl chloroformate

-
-
56613-80-0
(R)-Phenylglycinol

-
-
532986-19-9
hexyl (1R)-2-hydroxy-1-phenylethylcarbamate

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In 1,4-dioxane at 0℃; for 2h; | 99% |
-
-
56613-80-0
(R)-Phenylglycinol

-
-
2528-61-2
Heptanoic acid chloride

-
-
532986-14-4
N-[(1R)-2-hydroxy-1-phenylethyl]heptanamide

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In 1,4-dioxane at 0℃; for 2h; | 99% |
-
-
634-47-9
2-chloro-4-methylquinoline

-
-
56613-80-0
(R)-Phenylglycinol

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In isopropyl alcohol at 130℃; for 46h; | 99% |
-
-
17754-90-4
4-(Diethylamino)salicylaldehyde

-
-
56613-80-0
(R)-Phenylglycinol

| Conditions | Yield |
|---|---|
| In toluene for 8h; Heating; | 99% |
| In ethanol for 1h; Heating; | 95% |
D-Plenylglycinol Specification
The CAS registry number of D-Plenylglycinol is 56613-80-0. Its EINECS registry number is 260-287-5. The systematic name is (2R)-2-amino-2-phenylethanol. In addition, the molecular formula is C8H11NO. It is a kind of white to light yellow crystal powder and belongs to the classes of Pharmaceutical Intermediates; Alcohols and Derivatives. What's more, it should be stored in sealed container, and put in a cool and dry place.
Physical properties about this chemical are: (1)ACD/LogP: 0.42; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -2.6; (4)ACD/LogD (pH 7.4): -1.45; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 2; (10)#H bond donors: 3; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 12.47 Å2; (13)Index of Refraction: 1.572; (14)Molar Refractivity: 40.87 cm3; (15)Molar Volume: 124.1 cm3; (16)Polarizability: 16.2 ×10-24cm3; (17)Surface Tension: 48.8 dyne/cm; (18)Density: 1.104 g/cm3; (19)Flash Point: 125.3 °C; (20)Enthalpy of Vaporization: 52.69 kJ/mol; (21)Boiling Point: 261 °C at 760 mmHg; (22)Vapour Pressure: 0.00601 mmHg at 25°C.
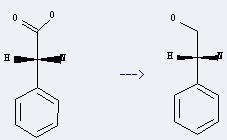
Preparation of D-Plenylglycinol: it can be prepared by (R)-amino-phenyl-acetic acid. This reaction will need reagents LiBH4 and trimethylsilyl chloride, and solvent tetrahydrofuran. The reaction time is 49 hours at reaction temperature of 0-20 °C. The yield is about 93%.
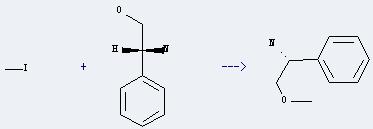
Uses of D-Plenylglycinol: it can react with iodomethane to get (R)-2-methoxy-1-phenylethylamine. This reaction is a kind of etherification reaction. The reaction will need reagent NaH and solvent tetrahydrofuran. The reaction time is 1 hour by heating.
When you are using this chemical, please be cautious about it as the following:
This chemical can cause burns. It is irritating to eyes, respiratory system and skin. And it is toxic by inhalation, in contact with skin and if swallowed. During using it, wear suitable protective clothing, gloves and eye/face protection and avoid contact with skin and eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. In addition, you should not breathe dust. Moreover, in case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: OC[C@H](N)c1ccccc1
(2)InChI: InChI=1/C8H11NO/c9-8(6-10)7-4-2-1-3-5-7/h1-5,8,10H,6,9H2/t8-/m0/s1
(3)InChIKey: IJXJGQCXFSSHNL-QMMMGPOBBC
Related Products
- D-Plenylglycinol
- 56613-81-1
- 56614-13-2
- 56614-57-4
- 566155-76-8
- 566158-47-2
- 56616-85-4
- 56616-91-2
- 566-17-6
- 56619-23-9
- 566-19-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View