-
Name
DIETHYLALUMINUM ETHOXIDE
- EINECS
- CAS No. 1586-92-1
- Article Data9
- CAS DataBase
- Density g/cm3
- Solubility
- Melting Point 2.5-4.5oC
- Formula C6H15 Al O
- Boiling Point °Cat760mmHg
- Molecular Weight 130.166
- Flash Point °C
- Transport Information
- Appearance
- Safety Ignites spontaneously in air in the pure form or in solutions with concentrations greater than 20%. When heated to decomposition it emits acrid smoke and fumes. See also ALUMINUM COMPOUNDS.
- Risk Codes R11;R14/15;R17;R20;R34;
-
Molecular Structure
-
Hazard Symbols
F
C
- Synonyms (Ethanolato)diethylaluminum;Dealox; Diethylaluminium ethoxide; Diethylaluminum ethoxide; Diethylaluminummonoethoxide; Diethylethoxyaluminum; Ethoxydiethylaluminum
- PSA 9.23000
- LogP 2.43500
Synthetic route

| Conditions | Yield |
|---|---|
| at 170℃; |
-
-
97-93-8
triethylaluminum

-
-
141-52-6
sodium ethanolate

-
A
-
1586-92-1
diethyl(ethoxy)aluminum

-
B
-
2397-68-4
sodium tetraethylaluminate

| Conditions | Yield |
|---|---|
| In hexane; n-heptane; toluene at 70℃; for 1h; |


| Conditions | Yield |
|---|---|
| With ethanol In hexane byproducts: ethane; Ar, ethanol in hexane added dropwise to a soln. of AlEt3 at -30°C; hexane was distilled off; residue was vac. distilled; |

-
-
1586-92-1
diethyl(ethoxy)aluminum

| Conditions | Yield |
|---|---|
| With O2 In xylene shaking of Al2Et6 soln. with different amts. of dry atm. O2 (mole ratio 3.5/1 - 2/1); spect. anal. {(27)Al-NMR}; | |
| With O2 In n-heptane shaking of Al2Et6 soln. with different amts. of dry atm. O2 (mole ratio 3.5/1 - 2/1); spect. anal. {(27)Al-NMR}; |

-
-
106475-72-3
methyltriphenylphosphonium bromide

-
-
1586-92-1
diethyl(ethoxy)aluminum

-
-
106-99-0
buta-1,3-diene

| Conditions | Yield |
|---|---|
| In further solvent(s) solvent: ethyleneoxide, addn. of (C6H5)2C(Br)CH3 to the react. mixt. at 0-10°C; | 99% |
| In further solvent(s) solvent: ethyleneoxide, addn. of (C6H5)2C(Br)CH3 to the react. mixt. at 0-10°C; | 99% |
| In diethyl ether an orange soln. reacts with phenyl- and halide substituted methane derivative; |


| Conditions | Yield |
|---|---|
| In diethyl ether -80°C, filtration, addn. of allylbromide to the mixt. at 0°C; | 95% |
| In diethyl ether -80°C, filtration, addn. of allylbromide to the mixt. at 0°C; | 95% |
| In diethyl ether byproducts: (π-C3H5NiBr)2; -80°C, filtration, addn. of allylbromide to the mixt. at -20°C; | |
| In diethyl ether byproducts: (π-C3H5NiBr)2; -80°C, filtration, addn. of allylbromide to the mixt. at -20°C; |


-
-
1586-92-1
diethyl(ethoxy)aluminum

-
-
5259-72-3, 10060-40-9, 111-78-4
1,5-dicyclooctadiene

-
-
1295-35-8
bis(1,5-cyclooctadiene)nickel (0)

| Conditions | Yield |
|---|---|
| In benzene molar ratio nickel acetylacetonat : cyclooctadiene = 1 : 2.5-5, benzene free from air, reduction between 0 and 5°C, 2 h, and room temp. 15 h; | 93.5% |
| In benzene molar ratio nickel acetylacetonate : cyclooctadiene = 1 : 2.5-5, benzene free from air, reduction between 0 and 5°C, 2 h, and room temp. 15 h; washing with cold benzene and ether; | 93.5% |
| In benzene molar ratio nickel acetylacetonate : cyclooctadiene = 1 : 2.5-5, benzene free from air, reduction between 0 and 5°C, 2 h, and room temp. 15 h; washing with cold benzene and ether; | 93.5% |
| In benzene molar ratio nickel acetylacetonat : cyclooctadiene = 1 : 2.5-5, benzene free from air, reduction between 0 and 5°C, 2 h, and room temp. 15 h; | 93.5% |


| Conditions | Yield |
|---|---|
| 100°C; | 93% |
-
-
852919-31-4
(Z)-1-chloro-4-phenylbut-1-en-1-yl acetate

-
-
1586-92-1
diethyl(ethoxy)aluminum

-
-
10031-93-3
ethyl 4-phenylbutyrate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 1h; Heating; | 92% |
-
-
366-18-7
[2,2]bipyridinyl

-
-
1586-92-1
diethyl(ethoxy)aluminum

-
-
15218-76-5
diethyl(2,2'-bipyridyl)nickel(II)

| Conditions | Yield |
|---|---|
| Stage #1: [2,2]bipyridinyl; bis(acetylacetonate)nickel(II) In diethyl ether at 25℃; Inert atmosphere; Schlenk technique; Stage #2: diethyl(ethoxy)aluminum In diethyl ether at -20 - 25℃; for 52h; Inert atmosphere; Schlenk technique; | 87% |

-
-
103-30-0
(E)-1,2-diphenyl-ethene

-
-
23777-40-4
bis(triphenylphosphine)ethylenenickel(0)

-
-
1586-92-1
diethyl(ethoxy)aluminum

-
-
12151-25-6, 53586-21-3
trans-C6H5CHCHC6H5Ni(P(C6H5)3)2

| Conditions | Yield |
|---|---|
| With triphenylphosphine In benzene byproducts: methylenecyclopropane, methylenecyclopropane trimers; Ar-atmosphere; molar ratio Ni-complex : olefin = 1:3, stirring (20°C, 22 h); evapn., pptn. with ether, filtration, washing (ether), drying (vac.); | 80% |


| Conditions | Yield |
|---|---|
| at 170℃; |
-
-
97-93-8
triethylaluminum

-
-
141-52-6
sodium ethanolate

-
A
-
1586-92-1
diethyl(ethoxy)aluminum

-
B
-
2397-68-4
sodium tetraethylaluminate

| Conditions | Yield |
|---|---|
| In hexane; n-heptane; toluene at 70℃; for 1h; |


| Conditions | Yield |
|---|---|
| With ethanol In hexane byproducts: ethane; Ar, ethanol in hexane added dropwise to a soln. of AlEt3 at -30°C; hexane was distilled off; residue was vac. distilled; |

-
-
1586-92-1
diethyl(ethoxy)aluminum

| Conditions | Yield |
|---|---|
| With O2 In xylene shaking of Al2Et6 soln. with different amts. of dry atm. O2 (mole ratio 3.5/1 - 2/1); spect. anal. {(27)Al-NMR}; | |
| With O2 In n-heptane shaking of Al2Et6 soln. with different amts. of dry atm. O2 (mole ratio 3.5/1 - 2/1); spect. anal. {(27)Al-NMR}; |

-
-
106475-72-3
methyltriphenylphosphonium bromide

-
-
1586-92-1
diethyl(ethoxy)aluminum

-
-
106-99-0
buta-1,3-diene

| Conditions | Yield |
|---|---|
| In further solvent(s) solvent: ethyleneoxide, addn. of (C6H5)2C(Br)CH3 to the react. mixt. at 0-10°C; | 99% |
| In further solvent(s) solvent: ethyleneoxide, addn. of (C6H5)2C(Br)CH3 to the react. mixt. at 0-10°C; | 99% |
| In diethyl ether an orange soln. reacts with phenyl- and halide substituted methane derivative; |


| Conditions | Yield |
|---|---|
| In diethyl ether -80°C, filtration, addn. of allylbromide to the mixt. at 0°C; | 95% |
| In diethyl ether -80°C, filtration, addn. of allylbromide to the mixt. at 0°C; | 95% |
| In diethyl ether byproducts: (π-C3H5NiBr)2; -80°C, filtration, addn. of allylbromide to the mixt. at -20°C; | |
| In diethyl ether byproducts: (π-C3H5NiBr)2; -80°C, filtration, addn. of allylbromide to the mixt. at -20°C; |


-
-
1586-92-1
diethyl(ethoxy)aluminum

-
-
5259-72-3, 10060-40-9, 111-78-4
1,5-dicyclooctadiene

-
-
1295-35-8
bis(1,5-cyclooctadiene)nickel (0)

| Conditions | Yield |
|---|---|
| In benzene molar ratio nickel acetylacetonat : cyclooctadiene = 1 : 2.5-5, benzene free from air, reduction between 0 and 5°C, 2 h, and room temp. 15 h; | 93.5% |
| In benzene molar ratio nickel acetylacetonate : cyclooctadiene = 1 : 2.5-5, benzene free from air, reduction between 0 and 5°C, 2 h, and room temp. 15 h; washing with cold benzene and ether; | 93.5% |
| In benzene molar ratio nickel acetylacetonate : cyclooctadiene = 1 : 2.5-5, benzene free from air, reduction between 0 and 5°C, 2 h, and room temp. 15 h; washing with cold benzene and ether; | 93.5% |
| In benzene molar ratio nickel acetylacetonat : cyclooctadiene = 1 : 2.5-5, benzene free from air, reduction between 0 and 5°C, 2 h, and room temp. 15 h; | 93.5% |


| Conditions | Yield |
|---|---|
| 100°C; | 93% |
-
-
852919-31-4
(Z)-1-chloro-4-phenylbut-1-en-1-yl acetate

-
-
1586-92-1
diethyl(ethoxy)aluminum

-
-
10031-93-3
ethyl 4-phenylbutyrate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 1h; Heating; | 92% |
-
-
366-18-7
[2,2]bipyridinyl

-
-
1586-92-1
diethyl(ethoxy)aluminum

-
-
15218-76-5
diethyl(2,2'-bipyridyl)nickel(II)

| Conditions | Yield |
|---|---|
| Stage #1: [2,2]bipyridinyl; bis(acetylacetonate)nickel(II) In diethyl ether at 25℃; Inert atmosphere; Schlenk technique; Stage #2: diethyl(ethoxy)aluminum In diethyl ether at -20 - 25℃; for 52h; Inert atmosphere; Schlenk technique; | 87% |

-
-
103-30-0
(E)-1,2-diphenyl-ethene

-
-
23777-40-4
bis(triphenylphosphine)ethylenenickel(0)

-
-
1586-92-1
diethyl(ethoxy)aluminum

-
-
12151-25-6, 53586-21-3
trans-C6H5CHCHC6H5Ni(P(C6H5)3)2

| Conditions | Yield |
|---|---|
| With triphenylphosphine In benzene byproducts: methylenecyclopropane, methylenecyclopropane trimers; Ar-atmosphere; molar ratio Ni-complex : olefin = 1:3, stirring (20°C, 22 h); evapn., pptn. with ether, filtration, washing (ether), drying (vac.); | 80% |

-
-
13423-15-9
3-methyltetrahydrofuran

-
-
13470-26-3
vanadium(III) bromide

-
-
143-66-8
sodium tetraphenyl borate

-
-
1586-92-1
diethyl(ethoxy)aluminum

| Conditions | Yield |
|---|---|
| In further solvent(s) (Ar); VBr3 and 3-methyltetrahydrofuran refluxed for 12 h; cooled (room temp.); addn. of soln. of AlEt2OEt in toluene; stirred (10 days; room temp.); filtration through Celite onto NaBPh4; stirred for 12 h at room temp.;; filtration (Celite); hexane placed on top of soln.; soln. allowed to stand for several days; crystals filtered, washed (hexane) and dried (vacuum);; | 76.5% |


| Conditions | Yield |
|---|---|
| In diethyl ether at about 0°C; recrystn. from ether; | 75% |
| In diethyl ether at about 0°C; | 57% |
| In diethyl ether at about 0°C; | 57% |

-
-
591-12-8
5-methyl-2-furanone

-
-
1586-92-1
diethyl(ethoxy)aluminum

-
-
100-52-7
benzaldehyde

-
-
95891-75-1
(4S,5S)-4-Acetyl-5-phenyl-dihydro-furan-2-one

-
-
95891-76-2
β-acetyl-γ-phenyl-γ-butyrolactone

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0℃; for 4h; crossed aldol reaction; | A 74% B n/a C n/a |


-
-
1586-92-1
diethyl(ethoxy)aluminum

| Conditions | Yield |
|---|---|
| In benzene 0-20°C; | 71% |
| In benzene 0-20°C; | 71% |
| In benzene 0-5°C; | 60% |
| In benzene 0-5°C; | 60% |


| Conditions | Yield |
|---|---|
| In diethyl ether from -20°C to 10°C, under N2, Ar or in vac.; | 70% |
| In diethyl ether from -20°C to 10°C, under N2, Ar or in vac.; | 50% |


-
-
1586-92-1
diethyl(ethoxy)aluminum

-
-
102150-17-4
(2,2'-bipyridine)diethylpalladium(II)

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl In diethyl ether under Ar; Pd(acac)2 and 2,2'-bipyridine were suspensed; addition of Al(C2H5)2(OC2H5); stirred for 4 d at 22°C; filtered; washed 3 times with Et2O; dried in vacuo; crystals extracted (acetone) under reduced pressure; solid separated; dried in vacuo; elem.anal.; | A 67% B n/a |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -10℃; for 4h; crossed aldol reaction; | A 61% B n/a C n/a |

-
-
109-99-9
tetrahydrofuran

-
-
13470-26-3
vanadium(III) bromide

-
-
143-66-8
sodium tetraphenyl borate

-
-
1586-92-1
diethyl(ethoxy)aluminum

| Conditions | Yield |
|---|---|
| In tetrahydrofuran (Ar); VBr3 and tetrahydrofuran refluxed for 12 h; cooled (room temp.); addn. of soln. of AlEt2OEt in toluene; stirred (10 days; room temp.); filtration through Celite onto NaBPh4; stirred for 12 h at room temp.;; filtration (Celite); hexane placed on top of soln.; soln. allowed to stand for several days; crystals filtered, washed (hexane) and dried (vacuum);; | 61% |

-
-
110546-20-8
2,2-[μ-(N,N'-piperazindiyl)dimethyl]-bis(4,6-di-tert-butyl-phenol)

-
-
1586-92-1
diethyl(ethoxy)aluminum

-
-
1214751-50-4
ethoxy aluminium-N,N-bis(benzyl-3,5-di-tert-butyl-phenol)piperazine

| Conditions | Yield |
|---|---|
| In diethyl ether; toluene in atm. of N2 or Ar using Schlenk technique; AlEt2OEt in Et2O added dropwise to piperazine deriv. soln. in toluene at 50°C, stirred at 50°C ca. 30 min; mixt. refluxed at 110°C for 5 h; solvent removed in vacuo, solid washed with pentane 3 times; pumped dry;elem. anal.; | 59% |

| Conditions | Yield |
|---|---|
| In diethyl ether under Ar; a mixt. of Fe-contg. compd. (12.9 mmol) and a ligand (30.3 mmol) in Et2O was treated dropwise at ca. -20°C with Al-contg. compd. (36.7 mmol); the mixt. was allowed to warm up to room temp.; stirring for 12 h at room temp.; the solid was filtered off, washed with Et2O, C7H16 and dried in vac.; IR-, NMR spectra; | 55% |

-
-
14024-18-1
iron(III)-acetylacetonate

-
-
1586-92-1
diethyl(ethoxy)aluminum

-
-
106-99-0
buta-1,3-diene

-
-
672-66-2
Dimethyl(phenyl)phosphine

-
-
88242-44-8
(1,3-butadiene)tris(dimetylphenylphosphine)iron(0)

| Conditions | Yield |
|---|---|
| In not given reaction of Fe(acac)3 with AlEt2OEt in the presence of PPhMe2, excess of 1,3-butadiene, 0°C; | 52% |


-
-
1586-92-1
diethyl(ethoxy)aluminum

-
-
6142-73-0
methylene cyclopropane

-
-
89606-46-2
(η2-methylenecyclopropane)bis(triphenylphosphine)nickel

| Conditions | Yield |
|---|---|
| With triphenylphosphine In benzene Ar-atmosphere; molar ratio Ni : PPh3 : Al : olefin = 1:2:3:4; stirring (20°C, 9 h, pptn.); filtration, washing (ether), drying (vac.); | 44% |

-
-
51177-89-0
diphenyl dicyclopentadienyl-zirconium

-
-
1586-92-1
diethyl(ethoxy)aluminum

-
-
169617-72-5
(C5H5)2Zr(C6H4Al(C2H5)2OC2H5)

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; toluene Ar or N2-atmosphere; 80°C (18 h); evapn. (vac., room temp.), toluene addn., evapn. (vac.), pptn. on pentane addn. (sonicating, 30 min), filtering, pptn. on concg., decanting, drying (vac.); elem. anal.; | 44% |

-
-
1586-92-1
diethyl(ethoxy)aluminum

| Conditions | Yield |
|---|---|
| In tetrahydrofuran under Ar to C14H10Mg*nTHF in THF stirring at 20°C added during 30 min dropwise CH3CH2OAl(C2H5)2H in THF; after standing 1-2 d warmed to50-60°C, filtrated warm; crystn. in any h at -20°C; filtrated, washed with cooled ether,dried at 20°C/1E-3 torr; elem. anal.; | 43% |

-
-
1586-92-1
diethyl(ethoxy)aluminum

| Conditions | Yield |
|---|---|
| In benzene suspn. of Ni-compd. in benzene is treated with the Al compd. at room temp. (under Ar) with stirring, further stirring for 3 d; filtn., evapn. (vac.), addn. of THF, filtn., addn. of ether to the filtrate and extn. with benzene; elem. anal.; | A 40% B n/a |

-
-
852919-31-4
(Z)-1-chloro-4-phenylbut-1-en-1-yl acetate

-
-
1586-92-1
diethyl(ethoxy)aluminum

| Conditions | Yield |
|---|---|
| With dimethyl sulfoxide In tetrahydrofuran at 0 - 20℃; for 15h; Claisen condensation; | 38% |

| Conditions | Yield |
|---|---|
| In diethyl ether addn. of 11.0mmol (C2H5O)Al(C2H5)2 to 3.6mmol Pd-compound and 7.2mmol bipyridine in 50ml ether, stirring at 22°C for 6d, filtration, extractn. of residue with ethylether, concn. of ether extract, crystn. on cooling to -18°C (7d);; filtration under Ar, washing with ether, drying in vac.; elem. anal.;; | 32% |


-
-
1586-92-1
diethyl(ethoxy)aluminum

-
B
-
134539-87-0, 62056-37-5
1-methyl-4-(3-phenyl-1-propen-1-yl)benzene

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 20℃; for 15h; | A 27% B 16% |

-
-
66-71-7
1,10-Phenanthroline

-
-
1586-92-1
diethyl(ethoxy)aluminum

-
-
109298-81-9
diethyl(1.10-phenanthroline)palladium(II)

| Conditions | Yield |
|---|---|
| In tetrahydrofuran addn. of 11.54mmol (C2H5O)Al(C2H5)2 to 3.84mmol Pd-compound and 7.88mmol phenanthroline in 30ml dry THF, stirring at room temp. for 160h, filtration, concn. of filtrate, crystn. on cooling to -18°C for several days; Ar atm.;; filtration under Ar, washing with ether, drying in vac.; elem. anal.;; | 17% |

-
-
14024-18-1
iron(III)-acetylacetonate

-
-
1586-92-1
diethyl(ethoxy)aluminum

-
-
672-66-2
Dimethyl(phenyl)phosphine

-
-
88326-00-5
bis(ethylene)tris(dimetylphenylphosphine)iron(0)

| Conditions | Yield |
|---|---|
| In diethyl ether addn. of AlEt2OEt to soln. of Fe(acac)3 and PPhMe2, -39°C, warmed up to -5°C, stirred 30min, concd. up, soln. cooled down to -78°C, pptn., under deoxygenated Ar; recrystn. from ether below 0°C under Ar; elem. anal.; | 16% |


| Conditions | Yield |
|---|---|
| With bis-(diphenylphosphino)-ethane In diethyl ether AlEt2(OEt) added to mixt. of Ni(acac)2 and diphosphine in Et2O at -50°C, stirred at -20°C for 10 h; ppt. sepd. by filtration, washed with Et2O, dried in vac.; elem. anal.; | 12% |


-
-
1586-92-1
diethyl(ethoxy)aluminum

-
-
5259-72-3, 10060-40-9, 111-78-4
1,5-dicyclooctadiene

| Conditions | Yield |
|---|---|
| In benzene byproducts: Al(C5H7O2)3; 0°C; molar ratio of NiC5H7O2)2:1,5-cyclooctadiene:(C2H5)2AlOC2H5=1:2.5:2.3; 2.5h; solvolyse with ethanol and 2nHCl below 5°C; distn. in high vac. at 40-50°C; cooling and crystn.; | 10% |


| Conditions | Yield |
|---|---|
| With Ni(acac); threophos In toluene at 40℃; for 48h; Yield given. Further byproducts given. Yields of byproduct given; |

Ethoxy diethyl aluminum Chemical Properties
Molecular Structure of Ethoxy diethyl aluminum (CAS NO.1586-92-1):
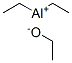
IUPAC: Ethoxy(diethyl)alumane
Molecular Formula:C6H15AlO
Molecular Weight:130.16
EINECS:216-447-1
Product Categories: Organometallics;AluminumChemical Synthesis;Micro/Nanoelectronics;Organoaluminum;Organometallic Reagents;Solution Deposition Precursors;Chemical Synthesis
Density:0.862 g/mL at 25oC
Melting Point:2.5-4.5oC(lit.)
Boiling Point:108-109oC
Flash Point:−1oF
Storage temp.:0-6oC
Vapor pressure:5 mm Hg (94oC)
Sensitive:Air & Moisture Sensitive
SMILES:CC[Al](CC)OCC
InChIKey:InChIKey: GCPCLEKQVMKXJM-XEYNOYNSAZ
Ethoxy diethyl aluminum Safety Profile
Ignites spontaneously in air in the pure form or in solutions with concentrations greater than 20%. When heated to decomposition it emits acrid smoke and fumes. See also ALUMINUM COMPOUNDS.
Safty informations about Ethoxy diethyl aluminum (CAS NO.1586-92-1):
Hazard Codes:F ,T
,T ,C
,C
Risk Statements:11-14-17-23/24/25-34-14/15
R11:Highly flammable.
R14 :Reacts violently with water.
R17:Spontaneously flammable in air.
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed.
R34:Causes burns.
R14/15:Reacts violently with water, liberating extremely flammable gases
Safety Statements:16-26-36/37/39-45-43-27
S16:Keep away from sources of ignition.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S43:In case of fire use ... (there follows the type of fire-fighting equipment to be used.)
S27:Take off immediately all contaminated clothing.
RIDADR:UN 3394 4.2/PG 1
HazardClass:4.3
PackingGroup:I
Ethoxy diethyl aluminum Specification
Ethoxy diethyl aluminum with CAS registry number of 1586-92-1 is also known as Aluminum,ethoxydiethyl- ; Dlethylaluminumethoxide ; Ethoxydiethyl-aluminu ; Ethoxydiethyl-aluminum ; Diethylaluminium ethoxide ; Diethylaluminum ethoxide ; Ethoxydiethylaluminium ; Diethylaluminum ethoxide,25 WT.% (1.6M )solution in toluene . It is an organometallic reagent with the appearance of colorless clear liquid. Ethoxy diethyl aluminum is used primarily as a catalyst component in
Ziegler-Natta type systems for olefin polymerizations. The pyrophoric nature of Ethoxy diethyl aluminum presents potential hazards not common to most liquid chemicals used by industry in tank truck quantities. Ethoxy diethyl aluminum, being pyrophoric, breaks into flame spontaneously and gives off dense smoke when exposed to air. It reacts violently with water. So this compoud must be kept away from water, acids, sparks, flames, and sources of ignition.
Related Products
- Ethoxy diethyl aluminum
- Ethoxy propionaldehyde
- Ethoxyacetic acid
- Ethoxyamine Hydrochloride
- Ethoxyaniline
- Ethoxybenzamide
- Ethoxycarbonyl isothiocyanate
- Ethoxycarbonyldigoxin
- Ethoxydiisobutylaluminium
- Ethoxydimethylvinylsilane
- 158702-89-7
- 1587-07-1
- 15871-58-6
- 15871-85-9
- 1587-20-8
- 15872-28-3
- 15872-41-0
- 15872-42-1
- 15872-44-3
- 158729-27-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View