-
Name
Fulvestrant
- EINECS 642-998-6
- CAS No. 129453-61-8
- Article Data21
- CAS DataBase
- Density 1.201 g/cm3
- Solubility
- Melting Point 104-106°C
- Formula C32H47F5O3S
- Boiling Point 674.8 °C at 760 mmHg
- Molecular Weight 606.781
- Flash Point 361.9 °C
- Transport Information
- Appearance white powder
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Faslodex;Estra-1,3,5(10)-triene-3,17-diol,7-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]-, (7a,17b)-;ICI 182780;ZD 182780;ZD 9238;ZM 182780;
- PSA 76.74000
- LogP 9.54830
Synthetic route
-
-
153004-31-0
(+)-(7α)-[9-(4,4,5,5,5-pentafluoropentylsulfanyl)nonyl]estra-1,3,5(10)-triene-3,17β-diol

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; acetic acid In water; ethyl acetate at 40℃; for 4h; Inert atmosphere; Schlenk technique; | 95% |
| Stage #1: (+)-(7α)-[9-(4,4,5,5,5-pentafluoropentylsulfanyl)nonyl]estra-1,3,5(10)-triene-3,17β-diol With β‐cyclodextrin In water; acetone at 20 - 60℃; for 0.5h; Stage #2: With N-Bromosuccinimide In water; acetone at 40℃; for 10h; Temperature; | 87% |
| With dihydrogen peroxide; acetic acid In water; ethyl acetate for 6 - 8h; | 85% |

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; acetic acid In ethyl acetate | 82% |
-
-
1221256-46-7
(7R,13S)-3-methoxy-13-methyl-7-(9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl)-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-17-ol

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride; thiourea In dichloromethane at 20 - 55℃; Product distribution / selectivity; | 32% |
-
-
261506-24-5
ICI 182,780-17β-acetate

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| With methanol; potassium hydroxide at 20℃; for 3h; Product distribution / selectivity; |

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water; acetic acid; ethyl acetate at 23℃; for 8h; Large scale reaction; | 88.4 kg |
-
-
148043-73-6
4,4,5,5,5-pentafluorpentan-1-ol

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: triethylamine / dichloromethane / 2 h / 0 - 20 °C / Inert atmosphere 2: isopropyl alcohol / 10 h / Reflux; Inert atmosphere 3: sodium hydroxide / acetonitrile; triethylamine; water / 3 h / 70 °C 4: sodium hydrogensulfate monohydrate / dichloromethane; water / 3 h / Reflux 5: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 2 h / 0 - 5 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: triphenylphosphine; di-isopropyl azodicarboxylate / tetrahydrofuran / 17 h / 0 - 20 °C 2.1: potassium tert-butylate / tetrahydrofuran; methanol / 70 °C 3.1: hydrogenchloride / dichloromethane; methanol; water / 1 h 3.2: Reflux 4.1: acetic acid; dihydrogen peroxide / water; ethyl acetate View Scheme | |
| Multi-step reaction with 4 steps 1: triethylamine; dmap / dichloromethane / 1 h / -10 °C 2: ethanol / 6 h / 65 °C 3: N,N-dimethyl-formamide / 20 °C 4: acetic acid; dihydrogen peroxide / ethyl acetate View Scheme |
-
-
252947-01-6
1-methanesulfonyloxy-4,4,5,5,5-pentafluoropentane

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: isopropyl alcohol / 10 h / Reflux; Inert atmosphere 2: sodium hydroxide / acetonitrile; triethylamine; water / 3 h / 70 °C 3: sodium hydrogensulfate monohydrate / dichloromethane; water / 3 h / Reflux 4: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 2 h / 0 - 5 °C View Scheme | |
| Multi-step reaction with 3 steps 1: ethanol / Reflux 2: sodium hydroxide / water; methanol / 24 h / Inert atmosphere; Reflux 3: sodium periodate / water; methanol / 24 h / 20 °C / Cooling with ice View Scheme |
-
-
434-22-0
19-nortestosterone

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: potassium acetate; oxygen / N,N-dimethyl-formamide / 12 h / 20 - 120 °C / 6000.6 Torr / Autoclave 2.1: trichlorophosphate / dichloromethane / 4 h / 35 °C 3.1: potassium tert-butylate / toluene / 1 h / 20 - 50 °C 3.2: 3 h / 10 - 100 °C 4.1: triethylsilane; boron trifluoride diethyl etherate / dichloromethane / 4 h / 0 - 25 °C 5.1: sodium hydroxide / acetonitrile; triethylamine; water / 3 h / 70 °C 6.1: sodium hydrogensulfate monohydrate / dichloromethane; water / 3 h / Reflux 7.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 2 h / 0 - 5 °C View Scheme | |
| Multi-step reaction with 7 steps 1.1: toluene-4-sulfonic acid / 1 h / Reflux 2.1: N-Bromosuccinimide / N,N-dimethyl-formamide; water / 1.25 h / 0 - 7 °C / Inert atmosphere 2.2: 2 h / 0 °C / Reflux; Inert atmosphere 3.1: copper(I) bromide / tetrahydrofuran / 0.75 h / -20 °C / Inert atmosphere 3.2: 0.5 h / -20 - 20 °C 4.1: copper(I) bromide; lithium bromide / acetonitrile / 24 h / 20 °C 5.1: water; sodium hydroxide / methanol / 5 h / 20 °C 6.1: 2,2-dimethoxy-2-phenylacetophenone / chloroform / 5 h / 20 °C / Inert atmosphere; UV-irradiation 7.1: acetic acid; dihydrogen peroxide / ethyl acetate / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 7 steps 1.1: toluene-4-sulfonic acid / 1 h / Reflux 2.1: N-Bromosuccinimide / N,N-dimethyl-formamide; water / 1.25 h / 0 - 7 °C / Inert atmosphere 2.2: 2 h / 0 °C / Reflux; Inert atmosphere 3.1: copper(I) bromide / tetrahydrofuran / 0.75 h / -20 °C / Inert atmosphere 3.2: 0.5 h / -20 - 20 °C 4.1: copper(I) bromide; lithium bromide / acetonitrile / 24 h / 20 °C 5.1: 2,2-dimethoxy-2-phenylacetophenone / chloroform / 5 h / 20 °C / Inert atmosphere; UV-irradiation 6.1: water; sodium hydroxide / methanol / 5 h / 20 °C 7.1: acetic acid; dihydrogen peroxide / ethyl acetate / 16 h / 20 °C View Scheme |
-
-
571-92-6
6-ketoestradiol

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: trichlorophosphate / dichloromethane / 4 h / 35 °C 2.1: potassium tert-butylate / toluene / 1 h / 20 - 50 °C 2.2: 3 h / 10 - 100 °C 3.1: triethylsilane; boron trifluoride diethyl etherate / dichloromethane / 4 h / 0 - 25 °C 4.1: sodium hydroxide / acetonitrile; triethylamine; water / 3 h / 70 °C 5.1: sodium hydrogensulfate monohydrate / dichloromethane; water / 3 h / Reflux 6.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 2 h / 0 - 5 °C View Scheme |

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: potassium tert-butylate / toluene / 1 h / 20 - 50 °C 1.2: 3 h / 10 - 100 °C 2.1: triethylsilane; boron trifluoride diethyl etherate / dichloromethane / 4 h / 0 - 25 °C 3.1: sodium hydroxide / acetonitrile; triethylamine; water / 3 h / 70 °C 4.1: sodium hydrogensulfate monohydrate / dichloromethane; water / 3 h / Reflux 5.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 2 h / 0 - 5 °C View Scheme |

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: triethylsilane; boron trifluoride diethyl etherate / dichloromethane / 4 h / 0 - 25 °C 2: sodium hydroxide / acetonitrile; triethylamine; water / 3 h / 70 °C 3: sodium hydrogensulfate monohydrate / dichloromethane; water / 3 h / Reflux 4: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 2 h / 0 - 5 °C View Scheme |

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium hydroxide / acetonitrile; triethylamine; water / 3 h / 70 °C 2: sodium hydrogensulfate monohydrate / dichloromethane; water / 3 h / Reflux 3: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 2 h / 0 - 5 °C View Scheme |

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium hydrogensulfate monohydrate / dichloromethane; water / 3 h / Reflux 2: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 2 h / 0 - 5 °C View Scheme |

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium hydroxide / acetonitrile; triethylamine; water / 3 h / 70 °C 2: sodium hydrogensulfate monohydrate / dichloromethane; water / 3 h / Reflux 3: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 2 h / 0 - 5 °C View Scheme |
-
-
875573-66-3
(+)-(7α)-[9-bromononyl]estra-1,3,5(10)-triene-3-ol-17β-yl acetate

-
-
148757-88-4
4,4,5,5,5-pentafluoro-1-pentanethiol

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: methanol / 2 h / 40 °C / Inert atmosphere; Schlenk technique 2: dihydrogen peroxide; acetic acid / water; ethyl acetate / 4 h / 40 °C / Inert atmosphere; Schlenk technique View Scheme |
-
-
160598-75-4
1-(acetylsulfanyl)-4,4,5,5,5-pentafluoropentane

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium hydroxide / methanol / 0.33 h / 20 °C / Inert atmosphere; Schlenk technique 2: methanol / 2 h / 40 °C / Inert atmosphere; Schlenk technique 3: dihydrogen peroxide; acetic acid / water; ethyl acetate / 4 h / 40 °C / Inert atmosphere; Schlenk technique View Scheme |
-
-
89359-54-6
9-bromo-1-nonene

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: Schwartz's reagent / dichloromethane / 0.33 h / 20 °C / Inert atmosphere; Schlenk technique 1.2: 0.17 h / 20 °C / Inert atmosphere; Schlenk technique 1.3: 20 °C / Inert atmosphere; Schlenk technique 2.1: lithium bromide; copper(ll) bromide / acetonitrile / 2 h / 20 °C / Inert atmosphere; Schlenk technique 3.1: methanol / 2 h / 40 °C / Inert atmosphere; Schlenk technique 4.1: dihydrogen peroxide; acetic acid / water; ethyl acetate / 4 h / 40 °C / Inert atmosphere; Schlenk technique View Scheme |
-
-
2590-41-2
6-dehydro-19-nortestosterone acetate

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: Schwartz's reagent / dichloromethane / 0.33 h / 20 °C / Inert atmosphere; Schlenk technique 1.2: 0.17 h / 20 °C / Inert atmosphere; Schlenk technique 1.3: 20 °C / Inert atmosphere; Schlenk technique 2.1: lithium bromide; copper(ll) bromide / acetonitrile / 2 h / 20 °C / Inert atmosphere; Schlenk technique 3.1: methanol / 2 h / 40 °C / Inert atmosphere; Schlenk technique 4.1: dihydrogen peroxide; acetic acid / water; ethyl acetate / 4 h / 40 °C / Inert atmosphere; Schlenk technique View Scheme | |
| Multi-step reaction with 5 steps 1.1: copper(I) bromide / tetrahydrofuran / 0.75 h / -20 °C / Inert atmosphere 1.2: 0.5 h / -20 - 20 °C 2.1: copper(I) bromide; lithium bromide / acetonitrile / 24 h / 20 °C 3.1: water; sodium hydroxide / methanol / 5 h / 20 °C 4.1: 2,2-dimethoxy-2-phenylacetophenone / chloroform / 5 h / 20 °C / Inert atmosphere; UV-irradiation 5.1: acetic acid; dihydrogen peroxide / ethyl acetate / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: copper(I) bromide / tetrahydrofuran / 0.75 h / -20 °C / Inert atmosphere 1.2: 0.5 h / -20 - 20 °C 2.1: copper(I) bromide; lithium bromide / acetonitrile / 24 h / 20 °C 3.1: 2,2-dimethoxy-2-phenylacetophenone / chloroform / 5 h / 20 °C / Inert atmosphere; UV-irradiation 4.1: water; sodium hydroxide / methanol / 5 h / 20 °C 5.1: acetic acid; dihydrogen peroxide / ethyl acetate / 16 h / 20 °C View Scheme |
-
-
875573-63-0
(+)-3-oxo-(7α)-[9-bromononyl]estr-4-en-17β-ylacetate

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: lithium bromide; copper(ll) bromide / acetonitrile / 2 h / 20 °C / Inert atmosphere; Schlenk technique 2: methanol / 2 h / 40 °C / Inert atmosphere; Schlenk technique 3: dihydrogen peroxide; acetic acid / water; ethyl acetate / 4 h / 40 °C / Inert atmosphere; Schlenk technique View Scheme | |
| Multi-step reaction with 4 steps 1: copper(ll) bromide; lithium bromide / acetonitrile / 3 h / 20 °C / Large scale 2: hydrogen bromide / methanol / 3 h / Reflux; Large scale 3: sodium hydroxide / N,N-dimethyl-formamide; water / 1.5 h / 0 - 15 °C / Large scale 4: acetic acid; dihydrogen peroxide / ethyl acetate / 8 h / 20 - 30 °C / Large scale View Scheme |

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: potassium tert-butylate / tetrahydrofuran; methanol / 70 °C 2.1: hydrogenchloride / dichloromethane; methanol; water / 1 h 2.2: Reflux 3.1: acetic acid; dihydrogen peroxide / water; ethyl acetate View Scheme |
-
-
862700-61-6
thiobenzoic acid S-(4,4,5,5,5-pentafluoro-pentyl)ester

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: potassium tert-butylate / tetrahydrofuran; methanol / 70 °C 2.1: hydrogenchloride / dichloromethane; methanol; water / 1 h 2.2: Reflux 3.1: acetic acid; dihydrogen peroxide / water; ethyl acetate View Scheme |
-
-
122566-22-7
(8R,9S,13S,14S,17S)-13-Methyl-3,17-bis-(tetrahydro-pyran-2-yloxy)-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-6-ol

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: pyridine; Dess-Martin periodane / dichloromethane / 1 h / 20 °C 2.1: triethyl borane; potassium tert-butylate / toluene; tetrahydrofuran / 0.17 h / 110 °C 2.2: Reflux 3.1: potassium tert-butylate / tetrahydrofuran; methanol / 70 °C 4.1: hydrogenchloride / dichloromethane; methanol; water / 1 h 4.2: Reflux 5.1: acetic acid; dihydrogen peroxide / water; ethyl acetate View Scheme |
-
-
53573-82-3
3,17β-bis(2-tetrahydropyranyloxy)estra-1,3,5(10)-triene-6-one

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: triethyl borane; potassium tert-butylate / toluene; tetrahydrofuran / 0.17 h / 110 °C 1.2: Reflux 2.1: potassium tert-butylate / tetrahydrofuran; methanol / 70 °C 3.1: hydrogenchloride / dichloromethane; methanol; water / 1 h 3.2: Reflux 4.1: acetic acid; dihydrogen peroxide / water; ethyl acetate View Scheme |

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: copper(I) bromide; lithium bromide / acetonitrile / 24 h / 20 °C 2: water; sodium hydroxide / methanol / 5 h / 20 °C 3: 2,2-dimethoxy-2-phenylacetophenone / chloroform / 5 h / 20 °C / Inert atmosphere; UV-irradiation 4: acetic acid; dihydrogen peroxide / ethyl acetate / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1: copper(I) bromide; lithium bromide / acetonitrile / 24 h / 20 °C 2: 2,2-dimethoxy-2-phenylacetophenone / chloroform / 5 h / 20 °C / Inert atmosphere; UV-irradiation 3: water; sodium hydroxide / methanol / 5 h / 20 °C 4: acetic acid; dihydrogen peroxide / ethyl acetate / 16 h / 20 °C View Scheme |

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: water; sodium hydroxide / methanol / 5 h / 20 °C 2: 2,2-dimethoxy-2-phenylacetophenone / chloroform / 5 h / 20 °C / Inert atmosphere; UV-irradiation 3: acetic acid; dihydrogen peroxide / ethyl acetate / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 2,2-dimethoxy-2-phenylacetophenone / chloroform / 5 h / 20 °C / Inert atmosphere; UV-irradiation 2: water; sodium hydroxide / methanol / 5 h / 20 °C 3: acetic acid; dihydrogen peroxide / ethyl acetate / 16 h / 20 °C View Scheme |

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 2,2-dimethoxy-2-phenylacetophenone / chloroform / 5 h / 20 °C / Inert atmosphere; UV-irradiation 2: acetic acid; dihydrogen peroxide / ethyl acetate / 16 h / 20 °C View Scheme |

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: water; sodium hydroxide / methanol / 5 h / 20 °C 2: acetic acid; dihydrogen peroxide / ethyl acetate / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: acetic acid; dihydrogen peroxide / ethyl acetate / 0 - 25 °C 2: sodium hydroxide / methanol / 25 °C View Scheme |
-
-
148757-88-4
4,4,5,5,5-pentafluoro-1-pentanethiol

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 2,2-dimethoxy-2-phenylacetophenone / chloroform / 5 h / 20 °C / Inert atmosphere; UV-irradiation 2: water; sodium hydroxide / methanol / 5 h / 20 °C 3: acetic acid; dihydrogen peroxide / ethyl acetate / 16 h / 20 °C View Scheme |
-
-
4999-76-2
3,17β-diacetoxy-estra-3,5-diene

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: N-Bromosuccinimide / N,N-dimethyl-formamide; water / 1.25 h / 0 - 7 °C / Inert atmosphere 1.2: 2 h / 0 °C / Reflux; Inert atmosphere 2.1: copper(I) bromide / tetrahydrofuran / 0.75 h / -20 °C / Inert atmosphere 2.2: 0.5 h / -20 - 20 °C 3.1: copper(I) bromide; lithium bromide / acetonitrile / 24 h / 20 °C 4.1: water; sodium hydroxide / methanol / 5 h / 20 °C 5.1: 2,2-dimethoxy-2-phenylacetophenone / chloroform / 5 h / 20 °C / Inert atmosphere; UV-irradiation 6.1: acetic acid; dihydrogen peroxide / ethyl acetate / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 6 steps 1.1: N-Bromosuccinimide / N,N-dimethyl-formamide; water / 1.25 h / 0 - 7 °C / Inert atmosphere 1.2: 2 h / 0 °C / Reflux; Inert atmosphere 2.1: copper(I) bromide / tetrahydrofuran / 0.75 h / -20 °C / Inert atmosphere 2.2: 0.5 h / -20 - 20 °C 3.1: copper(I) bromide; lithium bromide / acetonitrile / 24 h / 20 °C 4.1: 2,2-dimethoxy-2-phenylacetophenone / chloroform / 5 h / 20 °C / Inert atmosphere; UV-irradiation 5.1: water; sodium hydroxide / methanol / 5 h / 20 °C 6.1: acetic acid; dihydrogen peroxide / ethyl acetate / 16 h / 20 °C View Scheme |

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: ethanol / 6 h / 65 °C 2: N,N-dimethyl-formamide / 20 °C 3: acetic acid; dihydrogen peroxide / ethyl acetate View Scheme |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid Heating; | 100% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dichloromethane at 0 - 20℃; for 2.5h; Schotten-Baumann reaction; | 99% |
| With potassium hydroxide In dichloromethane Esterification; |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid for 6h; Heating; | 97% |
-
-
129453-61-8
fulvestrant

-
-
229625-50-7
di-tert-butyl chloromethyl phosphate

| Conditions | Yield |
|---|---|
| Stage #1: fulvestrant With potassium tert-butylate In tetrahydrofuran at 70℃; for 0.0833333h; Inert atmosphere; Stage #2: di-tert-butyl chloromethyl phosphate In tetrahydrofuran at 70℃; | 95.38% |
-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| With fluorosulfonyl fluoride; triethylamine In acetonitrile at 20℃; for 1h; chemoselective reaction; | 90% |
| With fluorosulfonyl fluoride; triethylamine In dichloromethane at 20℃; for 12h; Sealed tube; | 72% |
-
-
103366-74-1
2,3,4,6-Tetra-O-pivaloyl-α-D-glucopyranosyl trichloroacetimidate

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Stage #1: fulvestrant With boron trifluoride diethyl etherate In 1,2-dichloro-ethane at -20℃; Stage #2: 2,3,4,6-Tetra-O-pivaloyl-α-D-glucopyranosyl trichloroacetimidate In 1,2-dichloro-ethane Further stages.; | 80% |
-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| With oxalyl dichloride; dimethyl sulfoxide; diisopropylamine In 1,2-dichloro-ethane at -70 - 20℃; Swern oxidation; | 65% |
-
-
990-91-0
dibenzyl [[bis(benzyloxy)phosphoryl]oxy]phosphonate

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Stage #1: fulvestrant With potassium tert-butylate In tetrahydrofuran at 70℃; for 0.0833333h; Stage #2: dibenzyl [[bis(benzyloxy)phosphoryl]oxy]phosphonate In tetrahydrofuran at 70℃; for 18h; | 62.99% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 48h; | 58% |
-
-
108-30-5
succinic acid anhydride

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| With dmap; 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 50℃; for 16h; | 54.08% |
-
-
5070-13-3
bis-(p-nitrophenyl) carbonate

-
-
13518-40-6
L-valine tert-butylester hydrochloride

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Stage #1: bis-(p-nitrophenyl) carbonate; L-valine tert-butylester hydrochloride In tetrahydrofuran at 0 - 20℃; Inert atmosphere; Stage #2: fulvestrant With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; | 48.8% |

-
-
1118-68-9
dimethylaminoacetic acid

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Stage #1: dimethylaminoacetic acid; fulvestrant With dmap In dichloromethane at 0℃; for 0.166667h; Stage #2: With dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 16h; | 46.77% |
-
-
5070-13-3
bis-(p-nitrophenyl) carbonate

-
-
16874-17-2
L-phenylalanine tert-butyl ester

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Stage #1: bis-(p-nitrophenyl) carbonate; L-phenylalanine tert-butyl ester With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 0 - 20℃; Inert atmosphere; Stage #2: fulvestrant With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; Inert atmosphere; | 33.96% |

-
-
3235-69-6
morpholin-4-ylacetic acid

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Stage #1: morpholin-4-ylacetic acid; fulvestrant With dmap In dichloromethane at 0℃; for 0.0833333h; Stage #2: With dicyclohexyl-carbodiimide In dichloromethane at 0℃; for 2h; | 33.07% |
-
-
39539-66-7
4-methyl-piperazine-1-carbonyl chloride

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Stage #1: fulvestrant With dmap; potassium carbonate In acetonitrile at 20℃; for 0.5h; Stage #2: 4-methyl-piperazine-1-carbonyl chloride at 0 - 20℃; for 18h; | 31.46% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 48h; | 27.7% |
-
-
4497-04-5
3-morpholin-4-ylpropionic acid

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 6h; | 26.37% |
-
-
129453-61-8
fulvestrant

-
-
229625-50-7
di-tert-butyl chloromethyl phosphate

| Conditions | Yield |
|---|---|
| Stage #1: fulvestrant With potassium tert-butylate In tetrahydrofuran at 70℃; for 0.25h; Stage #2: di-tert-butyl chloromethyl phosphate In tetrahydrofuran at 70℃; for 7h; | 26.14% |
-
-
814-49-3
diethyl chlorophosphate

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| Stage #1: fulvestrant With tetrabutylammomium bromide; sodium hydroxide In chloroform; water at 20℃; for 0.5h; Stage #2: diethyl chlorophosphate In chloroform; water at 20℃; for 16h; | 24.5% |
-
-
129453-61-8
fulvestrant

-
-
54699-92-2
(4-Methyl-piperazin-1-yl)-acetic acid

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 20℃; for 16h; | 24.37% |
-
-
37386-15-5
2-(pyrrolidin-1-yl)acetic acid

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 6h; | 23.24% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 48h; | 23.2% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 48h; | 22% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 48h; | 21.5% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 48h; | 21.1% |
-
-
129453-61-8
fulvestrant

-
-
69-72-7
salicylic acid

| Conditions | Yield |
|---|---|
| Stage #1: fulvestrant; salicylic acid With dmap In dichloromethane at 0℃; for 0.0833333h; Stage #2: With dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 16h; | 18.92% |
-
-
6300-04-5
3-dimethylaminopropionic acid

-
-
129453-61-8
fulvestrant

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 12h; | 18.62% |
Fulvestrant Chemical Properties
Structure of Fulvestrant (CAS NO.129453-61-8):
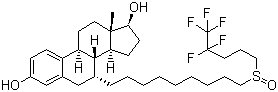
IUPAC Name: (7R,8R,9S,13S,14S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl]-7,8,9,11,12,14,15,16,
17-decahydrocyclopenta[a]phenanthrene-3,17-diol
Molecular Formula: C32H47F5O3S
Molar Mass: 606.77 g/mol
Flash Point: 361.9 °C
Boiling Point: 674.8°C at 760 mmHg
Vapour Pressure: 3.95E-19 mmHg at 25 °C
Density: 1.201 g/cm3
Storage Temp.: 2-8 °C
Index of Refraction: 1.521
Molar Refractivity: 154.04 cm3
Molar Volume: 505.1 cm3
Surface Tension: 41.9 dyne/cm
XLogP3: 9.2
H-Bond Donor: 2
H-Bond Acceptor: 8
Rotatable Bond Count: 14
Tautomer Count: 9
Exact Mass: 606.316607
MonoIsotopic Mass: 606.316607
Topological Polar Surface Area: 57.5
Heavy Atom Count: 41
Canonical SMILES: CC12CCC3C(C1CCC2O)C(CC4=C3C=CC(=C4)O)CCCCCCCCCS(=O)CCCC(C(F)(F)F)(F)F
Isomeric SMILES: C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@@H](CC4=C3C=CC(=C4)O)CCCCCCCCCS(=O)CCCC(C(F)(F)F)(F)F
InChI: InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-27+,28+,29-,30+,41/m1/s1
InChIKey: VWUXBMIQPBEWFH-WCCTWKNTSA-N
Product Categories: Intermediates & Fine Chemicals; Pharmaceuticals; Nuclear Receptors
Fulvestrant Uses
Fulvestrant (CAS NO.129453-61-8) is indicated for the treatment of hormone receptor positive metastatic breast cancer in postmenopausal women with disease progression following anti-estrogen therapy.
Fulvestrant Safety Profile
RTECS: KG7623000
Fulvestrant Specification
Fulvestrant (CAS NO.129453-61-8) is also named as 7alpha-(9-((4,4,5,5,5-Pentafluoropentyl)sulfinyl)nonyl)estra-1,3,5(10)-triene-3,17beta-diol ; CCRIS 8741 ; Faslodex ; HSDB 7658 ; ICI 182,780 ; ICI 182780 ; UNII-22X328QOC4 ; ZM 182780 . Fulvestrant (CAS NO.129453-61-8) is white powder.
Related Products
- Fulvestrant
- 129460-09-9
- 129-46-4
- 1294-74-2
- 1294-75-3
- 129477-21-0
- 129488-10-4
- 129488-34-2
- 129496-10-2
- 129-49-7
- 129497-78-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View