-
Name
Perchloroethane
- EINECS 200-666-4
- CAS No. 67-72-1
- Article Data390
- CAS DataBase
- Density 1.821 g/cm3
- Solubility 0.05 g/L (22 °C) in water
- Melting Point 184 °C
- Formula C2Cl6
- Boiling Point 186.8 °C at 760 mmHg
- Molecular Weight 236.74
- Flash Point 61.3 °C
- Transport Information UN 9037
- Appearance white crystalline powder
- Safety 36/37-61-45-36/37/39-26-24-16-7
- Risk Codes 40-51/53-36/37/38-39/23/24/25-36/38-23/24/25-11
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  N,
N,  T,
T,  F
F
- Synonyms Phenohep;Ethane,hexachloro- (8CI,9CI);1,1,1,2,2,2-Hexachloroethane;1,2-Dichloro-1,1,2,2-tetrachloroethane;Avlothane;Distokal;Distopan;Distopin;Egitol;Ethane hexachloride;Falkitol;Fasciolin;Fron 110;Hexachlorethane;Mottenhexe;NSC 9224;Perchloroethane;
- PSA 0.00000
- LogP 3.72680
Synthetic route
-
-
10170-69-1
dimanganese decacarbonyl

-
A
-
14100-30-2
pentacarbonylchloromanganese(I)

-
B
-
67-72-1
hexachloroethane

| Conditions | Yield |
|---|---|
| With tetrachloromethane In tetrachloromethane byproducts: CCl3; Irradiation (UV/VIS); Irradiation λ >350 nm, CCl4, Ar atm.;; detected by IR spectra and GCA;; | A n/a B 100% |

-
B
-
67-72-1
hexachloroethane

| Conditions | Yield |
|---|---|
| With tetrachloromethane In tetrachloromethane Irradiation (UV/VIS); Irradiation λ >490 nm, CCl4, Ar atm.;; detected by IR spectra and GCA;; | A n/a B 100% |
| With tetrachloromethane In tetrachloromethane; acetone Irradiation (UV/VIS); Irradiation λ >490 nm, acetone:CCl4=3:1, Ar atm.;; detected by IR spectra and GCA;; | A >99 B 70% |

-
-
67-72-1
hexachloroethane

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); chlorine at 130℃; under 11251.1 Torr; Reagent/catalyst; Temperature; Pressure; | 99% |

-
A
-
119-61-9
benzophenone

-
B
-
67-72-1
hexachloroethane

-
C
-
1885-81-0
p-chlorophenyl isocyanide

-
D
-
983-79-9
benzophenone azine

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A n/a B n/a C 98% D n/a |


| Conditions | Yield |
|---|---|
| With carbon monoxide In further solvent(s) Irradiation (UV/VIS); Irradiation of complex at 350-380 nm under 20 atm of CO in CBrCl3;; | A 81-87 B 97% C 78-85 D <4 E >65 |

-
-
56-23-5
tetrachloromethane

-
-
118334-80-8
N-acyloxyphthalimide

-
A
-
136918-14-4
phthalimide

-
B
-
67-72-1
hexachloroethane

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane In water; tert-butyl alcohol for 3h; Product distribution; Quantum yield; Irradiation; ratio, without DABCO; | A 95% B 70% C 74% D n/a |

-
-
77743-10-3
(2,5,6-trimethylbenzyl)bis(dimethylglyoximato)pyridinecobalt(III)

-
-
75-62-7
Bromotrichloromethane

-
B
-
67-72-1
hexachloroethane

-
C
-
54757-29-8
2-(Bromomethyl)-1,3,4-trimethylbenzene

| Conditions | Yield |
|---|---|
| In chloroform Excess of CHCl3, 50°C for 5 h;; detected by NMR spectra and GLC;; | A n/a B n/a C 5% D 95% |

-
-
56-23-5
tetrachloromethane

-
-
84784-54-3
1,4-diphenyl-2,3-benzo-7,7,8,8-tetramethyl-7,8-digermabicyclo<2,2,2>octadiene

-
A
-
67-72-1
hexachloroethane

-
B
-
796-30-5
1,4-diphenylnaphthalene

-
C
-
22702-77-8
1,2-dichlorotetramethyldigermane

-
D
-
1529-48-2
dichlorodimethylgermanium

| Conditions | Yield |
|---|---|
| In benzene Irradiation (UV/VIS); UV irradn. of a soln. of germanium compd. and CCl4 in C6H6 in a degassed sealed Pyrex tube for 3 h at room temp.; not isolated, detected by NMR, analyzed by GC and GC-MS; | A 38% B 95% C 71% D 27% |

-
-
115975-16-1
C24H22N2O3

-
A
-
119-61-9
benzophenone

-
B
-
67-72-1
hexachloroethane

-
C
-
84967-87-3
N-(β-Chlor-isopropyl)benzamid

-
D
-
983-79-9
benzophenone azine

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A n/a B n/a C 92% D n/a |

-
-
93845-09-1
C26H19NO2

-
A
-
119-61-9
benzophenone

-
B
-
67-72-1
hexachloroethane

-
C
-
983-79-9
benzophenone azine

-
D
-
2051-62-9
4'-biphenyl chloride

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A n/a B n/a C n/a D 88% |

-
-
115975-21-8
C24H17NO2

-
A
-
119-61-9
benzophenone

-
B
-
67-72-1
hexachloroethane

-
C
-
91-58-7
2-chloronaphthalene

-
D
-
983-79-9
benzophenone azine

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A n/a B n/a C 87% D n/a |

-
-
56-23-5
tetrachloromethane

-
-
595-90-4
tetraphenyltin(IV)

-
-
94-36-0
dibenzoyl peroxide

-
A
-
67-72-1
hexachloroethane

-
B
-
7646-78-8
tin(IV) chloride

-
C
-
108-90-7
chlorobenzene

-
D
-
88-99-3
benzene-1,2-dicarboxylic acid

-
E
-
65-85-0
benzoic acid

| Conditions | Yield |
|---|---|
| 50h boiling; | A n/a B 84.1% C n/a D n/a E n/a |


| Conditions | Yield |
|---|---|
| With photoreduced H2W10O324- In acetonitrile at 60℃; for 24h; Product distribution; Mechanism; other halocarbons, other redox-active polyoxotungstate complexes; other temperature, also with irradiation; other solvents; | A 82% B 2% |
| With pentaerythritol tetracaproate; dibenzoyl peroxide at 99.9℃; Product distribution; Rate constant; effect of concentration of pentaerythritol tetracaproate; dependence of rate constant from the number of (CH2) fragments in the molecule of esters; |
-
-
115975-11-6
diphenylmethanone O-(4-phenylbutanoyl) oxime

-
A
-
119-61-9
benzophenone

-
B
-
67-72-1
hexachloroethane

-
C
-
104-52-9
phenylpropyl chloride

-
D
-
983-79-9
benzophenone azine

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A n/a B n/a C 82% D n/a |

-
-
107264-20-0
diphenylmethanone O-(adamantane-1-carbonyl) oxime

-
A
-
119-61-9
benzophenone

-
B
-
67-72-1
hexachloroethane

-
C
-
935-56-8
1-chloroadamantane

-
D
-
983-79-9
benzophenone azine

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A n/a B n/a C 82% D n/a |

-
-
120569-16-6
C31H45NO2

-
A
-
119-61-9
benzophenone

-
B
-
67-72-1
hexachloroethane

-
C
-
62016-75-5
1-chloroheptadecane

-
D
-
983-79-9
benzophenone azine

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A n/a B n/a C 82% D n/a |

-
-
30435-62-2
C19H14N2O2

-
A
-
626-60-8
3-Chloropyridine

-
B
-
119-61-9
benzophenone

-
C
-
67-72-1
hexachloroethane

-
D
-
983-79-9
benzophenone azine

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A 82% B n/a C n/a D n/a |

-
-
120569-19-9
C37H49NO3

-
A
-
119-61-9
benzophenone

-
B
-
67-72-1
hexachloroethane

-
C
-
120577-31-3
(3R,5S,8R,9S,10S,13R,14S,17R)-17-((R)-3-Chloro-1-methyl-propyl)-10,13-dimethyl-hexadecahydro-cyclopenta[a]phenanthren-3-ol

-
D
-
983-79-9
benzophenone azine

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A n/a B n/a C 81% D n/a |

-
-
14688-34-7
Benzophenon-O-(p-chlorbenzoyl)-oxim

-
A
-
119-61-9
benzophenone

-
B
-
106-46-7
para-dichlorobenzene

-
C
-
67-72-1
hexachloroethane

-
D
-
983-79-9
benzophenone azine

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A n/a B 80% C n/a D n/a |

-
-
115975-20-7
C24H17NO2

-
A
-
119-61-9
benzophenone

-
B
-
67-72-1
hexachloroethane

-
C
-
983-79-9
benzophenone azine

-
D
-
90-13-1
1-Chloronaphthalene

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A n/a B n/a C n/a D 80% |

-
-
115993-91-4
C23H16N2O2

-
A
-
611-35-8
4-chloroquinoline

-
B
-
119-61-9
benzophenone

-
C
-
67-72-1
hexachloroethane

-
D
-
983-79-9
benzophenone azine

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A 80% B n/a C n/a D n/a |

-
-
120569-17-7
C35H53NO2

-
A
-
119-61-9
benzophenone

-
B
-
67-72-1
hexachloroethane

-
C
-
66326-16-7
1-chloroheneicosane

-
D
-
983-79-9
benzophenone azine

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A n/a B n/a C 80% D n/a |

-
-
93845-11-5
C19H14N2O2

-
A
-
109-09-1
2-chloropyridine

-
B
-
119-61-9
benzophenone

-
C
-
67-72-1
hexachloroethane

-
D
-
983-79-9
benzophenone azine

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A 80% B n/a C n/a D n/a |

-
-
6430-25-7
acetyl-triphenylgermane

-
A
-
67-72-1
hexachloroethane

-
B
-
918-00-3
1,1,1-trichloroacetone

-
C
-
1626-24-0
chlorotriphenylgermane

-
D
-
75-36-5
acetyl chloride

| Conditions | Yield |
|---|---|
| With tetrachloromethane In tetrachloromethane Irradiation (UV/VIS); Ph3GeCOMe photolyzed in CCl4; | A 53% B 10% C 78% D 74% |

-
-
120569-14-4
C23H16N2O2

-
A
-
1532-91-8
4-chloroisoquinoline

-
B
-
119-61-9
benzophenone

-
C
-
67-72-1
hexachloroethane

-
D
-
983-79-9
benzophenone azine

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A 77% B n/a C n/a D n/a |

-
-
93845-12-6
C19H14N2O2

-
A
-
626-61-9
4-Chloropyridine

-
B
-
119-61-9
benzophenone

-
C
-
67-72-1
hexachloroethane

-
D
-
983-79-9
benzophenone azine

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A 77% B n/a C n/a D n/a |

-
-
115975-18-3
C23H16N2O2

-
A
-
612-62-4
2-Chloroquinoline

-
B
-
119-61-9
benzophenone

-
C
-
67-72-1
hexachloroethane

-
D
-
983-79-9
benzophenone azine

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A 76% B n/a C n/a D n/a |

-
-
107264-22-2
C26H24N2O3

-
A
-
119-61-9
benzophenone

-
B
-
67-72-1
hexachloroethane

-
C
-
90670-04-5
(4-chloropiperidin-1-yl)(phenyl)methanone

-
D
-
983-79-9
benzophenone azine

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A n/a B n/a C 76% D n/a |

-
-
120569-15-5
C22H15N3O2

-
A
-
1448-87-9
2-chloroquinoxaline

-
B
-
119-61-9
benzophenone

-
C
-
67-72-1
hexachloroethane

-
D
-
983-79-9
benzophenone azine

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A 74% B n/a C n/a D n/a |

-
-
107264-24-4
C27H25NO3

-
A
-
119-61-9
benzophenone

-
B
-
67-72-1
hexachloroethane

-
C
-
107774-09-4
(2-chloro-cyclohexyl)-phenyl ketone

-
D
-
983-79-9
benzophenone azine

| Conditions | Yield |
|---|---|
| With tetrachloromethane Ambient temperature; Irradiation; Yields of byproduct given; | A n/a B n/a C 74% D n/a |

-
-
676491-10-4
4-(1-Phenyl-1-hydrazinocarbonyl-methylene)piperidine-1-carboxylic acid tert-butyl ester

-
-
67-72-1
hexachloroethane

-
-
1538-75-6
2,2-dimethylpropanoic anhydride

| Conditions | Yield |
|---|---|
| With IPr2NEt; triphenylphosphine In acetonitrile | 100% |


| Conditions | Yield |
|---|---|
| With LisBu In diethyl ether; cyclohexane (inert atm.); Ru complex in Et2O cooled to -78°C, treated with LisBu in cyclohexane (1:1.70) at -78°C within 20-30 s, stirred at -78°C for 3.5 h, treated with suspn. of ligand in Et2O, warmed to room temp. within 2.5 h, stirred for; evapd.(vac.), extd.(pentane), filtered (kieselghur), freed of volatiles,elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| With Oxone; II(H2O)2(Me2SO)4>(BF4)2; Hexadecyltrimethylammonium hydrogen sulfate In water at 20℃; Product distribution; other chloro- and bromoolefins; | 99% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: C2Cl4; Sonication; inert atmosphere; mass ratio metal : C2Cl6 = 1 : 3, 30 h; pptn. on pentane addn., decantation, washing (pentane), drying (room temp., dry box); elem. anal.; | 99% |

-
-
67-72-1
hexachloroethane

-
-
214750-14-8
Co(C5H4B(N(CH3)2)2)2

-
-
214750-16-0
Co(C5H4B(N(CH3)2)2)2(1+)*Cl(1-)=[Co(C5H4B(N(CH3)2)2)2]Cl

| Conditions | Yield |
|---|---|
| In toluene N2-atmosphere; stirring (4 h); filtering, drying (vac.), crystn. (CH2Cl2 / hexane = 1 : 1); elem. anal.; | 99% |
-
-
67-72-1
hexachloroethane

-
-
219944-90-8
5-(trimethylsilyl)-methyl-2,2′-bipyridine

-
-
219944-93-1
5-chloromethyl-2,2'-bipyridine

| Conditions | Yield |
|---|---|
| With cesium fluoride In acetonitrile at 60℃; for 4h; | 98% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether byproducts: PEt3HCl, Et3PO; under N2 for 12 h, filtration, adding of PMe3; react. time 10 min; filtration; elem. anal.; | 98% |


-
-
67-72-1
hexachloroethane

-
-
78251-21-5
W(6+)*CC(CH3)3(3-)*3Cl(1-)*OP(C2H5)3 = [W(CC(CH3)3)Cl3(PO(C2H5)3)]

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: PEt3HCl; under N2 for 10 h; filtration, pptn. of PEt3HCl with pentane, filtration, evapn.; | 98% |
-
-
67-72-1
hexachloroethane

-
-
214750-15-9
Co(C5H4B(N(C2H5)2)2)2

-
-
214750-17-1
Co(C5H4B(N(C2H5)2)2)2(1+)*Cl(1-)=[Co(C5H4B(N(C2H5)2)2)2]Cl

| Conditions | Yield |
|---|---|
| In toluene N2-atmosphere; stirring (4 h); filtering, drying (vac.), crystn. (CH2Cl2 / hexane = 1 : 1); elem. anal.; | 98% |

| Conditions | Yield |
|---|---|
| With n-butyllithium; sodium hydrogencarbonate In diethyl ether; water | 97.6% |

| Conditions | Yield |
|---|---|
| With aluminum(III) fluoride; hydrogen fluoride at 120℃; under 11251.1 Torr; for 10h; Reagent/catalyst; Temperature; Autoclave; | 97.06% |
| With CFC-112a; antimonypentachloride; fluorine at 32℃; unter vermindertem Druck; | |
| With antimonypentachloride; antimony(III) fluoride at 48℃; |

| Conditions | Yield |
|---|---|
| In tetrachloromethane at 300℃; for 0.000555556h; Temperature; Flow reactor; Pyrolysis; | 97% |
| at 600℃; | |
| With pyrographite at 700℃; |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: C2Cl4; Sonication; inert atmosphere; mass ratio metal : C2Cl6 = 1 : 3, 6 h; solvent evapn. (vac.), pentane addn., filtration, washing (pentane), drying (vac., 70 - 80°C, 10 - 15 min); elem. anal.; | 97% |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: C2Cl4; Sonication; inert atmosphere; mass ratio metal : C2Cl6 = 1 : 3, 45 h; pptn. on pentane addn., decantation, washing (pentane), drying (room temp., dry box); elem. anal.; | 96% |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: C2Cl4; Sonication; inert atmosphere; mass ratio metal : C2Cl6 = 1 : 3, 12 h; solvent evapn. (vac.), pentane addn., filtration, washing (pentane), drying (vac., 70 - 80°C, 10 - 15 min); elem. anal.; | 96% |

-
-
67-72-1
hexachloroethane

-
-
1261240-33-8
Rh((P(C6H5)2CH2)4N2)(1+)*BF4(1-)=[Rh((P(C6H5)2CH2)4N2)]BF4

-
-
1261240-39-4
RhCl2((P(C6H5)2CH2)4N2)(1+)*BF4(1-)=[RhCl2((P(C6H5)2CH2)4N2)]BF4

| Conditions | Yield |
|---|---|
| In dichloromethane under N2 or Ar; C2Cl6 added to soln. of rhodium complex in CH2Cl2, mixt. stirred at room temp. for 3 h; evapn. under vac., residue washed with hexanes; elem. anal.; | 96% |
-
-
67-72-1
hexachloroethane

-
-
3348-44-5
bis(N,N-dimethylamino)chlorophosphine

-
A
-
683-85-2
N,N-dimethylaminodichlorophosphane

-
B
-
5574-93-6
chlorotris(dimethylamino)phosphonium chloride

| Conditions | Yield |
|---|---|
| In diethyl ether for 480h; | A 90% B 95% |

-
-
67-72-1
hexachloroethane

-
-
219944-91-9
6’-[(trimethylsilyl)methyl]-2’,2-bipyridine

-
-
82740-65-6
6-(chloromethyl)-2,2’-bipyridine

| Conditions | Yield |
|---|---|
| With cesium fluoride In acetonitrile at 60℃; for 4h; | 95% |
-
-
67-72-1
hexachloroethane

-
-
302320-86-1
η6-(4-triisopropylsiloxymethyl-1-methoxymethoxybenzene)tricarbonylchromium(0)

-
-
410083-62-4, 409359-96-2, 410083-63-5
(+)-(S)-η6-(2-chloro-4-triisopropylsiloxymethyl-1-methoxymethoxybenzene)tricarbonylchromium(0)

| Conditions | Yield |
|---|---|
| With (-)-sparteine; n-butyllithium In diethyl ether N2; soln. of Cr-contg. compd. (4.3 mmol) in ether at -78°C was added to soln. of (-)-sparteine (3 equiv.) and n-BuLi (1.1 equiv.) in ether at the same temp.; stirring for 1 h and quenching with C2Cl6 (8.6 mmol); warming to -10°C overnight; treatment with water; the org. layer was washed twice with water and once with brine, dried (MgSO4) and concd. under reduced pressure; flash column chromy. (Sorbisil C-60; eluant: 10% ether-hexane); | 95% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 50℃; for 1h; Solvent; | 95% |
-
-
67-72-1
hexachloroethane

-
-
219944-89-5
4-(trimethylsilyl)-methyl-2,2'-bipyridine

-
-
219944-92-0
4-chloromethyl-2,2'-bipyridine

| Conditions | Yield |
|---|---|
| With cesium fluoride In acetonitrile at 60℃; for 4h; | 94% |
-
-
154671-38-2
bis[N,N-bistrimethylsilylamido]bis(tetrahydrofuran)ytterbium(II)

-
-
67-72-1
hexachloroethane

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 18h; Inert atmosphere; Glovebox; | 93% |
Hexachloroethane Chemical Properties
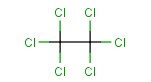
Molecular structure of Hexachloroethane (CAS NO.67-72-1) is:
Product Name: Hexachloroethane
CAS Registry Number: 67-72-1
IUPAC Name: 1,1,1,2,2,2-hexachloroethane
Molecular Weight: 236.7394 [g/mol]
Molecular Formula: C2Cl6
XLogP3: 4.1
EINECS: 200-666-4
Melting Point: 184 °C
Storage temp.: 2-8 °C
Water Solubility: 0.05 g/L (22 °C)
Surface Tension: 42.8 dyne/cm
Density: 1.821 g/cm3
Flash Point: 61.3 °C
Enthalpy of Vaporization: 40.57 kJ/mol
Boiling Point: 186.8 °C at 760 mmHg
Vapour Pressure: 0.895 mmHg at 25°C
Stability: Stable. Non-combustible. May react with hot metals, strong oxidizing agents.
Product Categories: refrigerants;Organics;Alpha Sort;E-LAlphabetic;H;HA -HT;Volatiles/ Semivolatiles
Hexachloroethane Uses
Hexachloroethane (CAS NO.67-72-1) is comonly used as raw materials for organic synthesis,and it also can be used as degassing agents of aluminum and its alloy.Also, it can be used in most aluminium foundries around the world to remove hydrogen.
Hexachloroethane Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LDLo | intravenous | 325mg/kg (325mg/kg) | Quarterly Journal of Pharmacy & Pharmacology. Vol. 7, Pg. 205, 1934. | |
| guinea pig | LD50 | oral | 4970mg/kg (4970mg/kg) | American Industrial Hygiene Association Journal. Vol. 40, Pg. 187, 1979. | |
| mouse | LD50 | intraperitoneal | 4500mg/kg (4500mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 11, Pg. 902, 1961. | |
| rabbit | LD | oral | > 1gm/kg (1000mg/kg) | American Industrial Hygiene Association Journal. Vol. 40, Pg. 187, 1979. | |
| rabbit | LD50 | skin | 32gm/kg (32000mg/kg) | American Industrial Hygiene Association Journal. Vol. 40, Pg. 187, 1979. | |
| rabbit | LDLo | subcutaneous | 4gm/kg (4000mg/kg) | Quarterly Journal of Pharmacy & Pharmacology. Vol. 7, Pg. 205, 1934. | |
| rat | LCLo | inhalation | 5900ppm/8H (5900ppm) | BEHAVIORAL: MUSCLE WEAKNESS | American Industrial Hygiene Association Journal. Vol. 40, Pg. 187, 1979. |
| rat | LD50 | oral | 4460mg/kg (4460mg/kg) | American Industrial Hygiene Association Journal. Vol. 40, Pg. 187, 1979. | |
| rat | LDLo | intraperitoneal | 2900mg/kg (2900mg/kg) | American Industrial Hygiene Association Journal. Vol. 40, Pg. 187, 1979. |
Hexachloroethane Safety Profile
Confirmed carcinogen with experimental carcinogenic data. A poison by intravenous route. Moderately toxic by intraperitoneal route. Mildly toxic by ingestion. Experimental reproductive effects. Mutation data reported. Liver injury has resulted from exposure to this material. An insecticide. Slightly explosive by spontaneous chemical reaction. Dehalogenation of this material by reaction with alkalies, metals, etc., will produce spontaneously explosive chloroacetylenes. When heated to decomposition it emits highly toxic fumes of Cl− and phosgene.
Hazard Codes:  Xn,
Xn,  N,
N,  T,
T,  F
F
Risk Statements: 40-51/53-36/37/38-39/23/24/25-36/38-23/24/25-11
R40:Limited evidence of a carcinogenic effect.
R51/53:Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
R36/37/38:Irritating to eyes, respiratory system and skin.
R39/23/24/25:Danger of very serious irreversible effects and Toxic by inhalation, in contact with skin and if swallowed.
R36/38:Irritating to eyes and skin.
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed.
R12:Extremely flammable.
Safety Statements: 36/37-61-45-36/37/39-26-24-16-7
S36/37:Wear suitable protective clothing and gloves.
S61:Avoid release to the environment. Refer to special instructions / safety data sheets.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S24:Avoid contact with skin.
S16:Keep away from sources of ignition.
S7:Keep container tightly closed.
RIDADR: UN 9037
WGK Germany: 3
RTECS: KI4025000
HazardClass: 9
PackingGroup: III
Hexachloroethane Specification
Hexachloroethane , its cas register number is 67-72-1. It also can be called 1,1,1,2,2,2-Hexachloroethane ; Avlothane ; Distokal ; Egitol ; Ethane hexachloride ; Ethane, hexachloro- ; Ethylene hexachloride ; Falkitol ; Hexachlor-aethan ; Mottenhexe ; Perchloroethane ; Phenohep ; Ethane, 1,1,1,2,2,2-hexachloro- .It is a colorless, crystalline solid with a camphor-like odor.It may cause illness from inhalation or ingestion and may irritate skin, eyes and mucous membranes. When heated to high temperatures it may emit toxic fumes. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. It insoluble in water.It can react with hot iron, zinc and aluminum.
Related Products
- Hexachloroethane
- 677-21-4
- 677-22-5
- 67724-03-2
- 67724-08-7
- 677-24-7
- 677275-33-1
- 677277-98-4
- 677297-55-1
- 677298-35-0
- 67730-10-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View