-
Name
Lilolidine
- EINECS 691-285-6
- CAS No. 5840-01-7
- Article Data14
- CAS DataBase
- Density 1.16 g/cm3
- Solubility
- Melting Point 80-81°C
- Formula C11H11 N
- Boiling Point 318.377 °C at 760 mmHg
- Molecular Weight 157.215
- Flash Point 146.349 °C
- Transport Information
- Appearance
- Safety 24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 1,7-Trimethyleneindol;1,8-Trimethyleneindole; 5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinoline; Indole,1,7-(1,3-propanediyl)-; Lilolidene
- PSA 4.93000
- LogP 2.58750
Synthetic route
-
-
124730-56-9
5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinoline-1-carboxylic acid

-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| With quinoline; copper chromite at 185℃; for 3h; | 93% |
| With quinoline; copper chromite at 185℃; for 2h; | 72% |
| With copper chromite In quinoline at 185℃; for 2h; | 72% |
| With quinoline; copper chromite at 185℃; for 4h; | 58% |
| copper(II) chromite In quinoline at 185℃; for 4h; | 58% |
-
-
16078-37-8
5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-2(1H)-one

-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| Stage #1: 5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-2(1H)-one With diisobutylaluminium hydride In tetrahydrofuran; toluene at -20 - -10℃; Stage #2: With hydrogenchloride In tetrahydrofuran; water; toluene at 40℃; for 0.5h; Temperature; Concentration; Reagent/catalyst; | 88.9% |
-
-
1220339-77-4
5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinoline 1,2-dicarboxylic acid

-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| Stage #1: 5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinoline 1,2-dicarboxylic acid With quinoline; copper at 238℃; for 3h; Stage #2: With hydrogenchloride In water Stage #3: With sodium hydroxide In water | 72% |
-
-
635-46-1
1,2,3,4-tetrahydroisoquinoline

-
-
107-21-1
ethylene glycol

-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| With Pt/Al2O3; zinc(II) oxide In neat (no solvent) at 175℃; for 24h; Sealed tube; | 50% |
-
-
635-46-1
1,2,3,4-tetrahydroisoquinoline

-
-
107-21-1
ethylene glycol

-
A
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

-
B
-
52704-48-0
2-[1,2,3,4-tetrahydroquinolin-1-yl]ethan-1-ol

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; zinc(II) oxide In water at 150℃; for 24h; Sealed tube; | A 45% B 45% |
| With palladium on activated charcoal; zinc(II) oxide In water at 150℃; for 24h; Sealed tube; |

-
-
91-22-5
quinoline

-
-
107-21-1
ethylene glycol

-
A
-
635-46-1
1,2,3,4-tetrahydroisoquinoline

-
B
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; zinc In water at 150℃; for 24h; Autoclave; | A 37% B 16% |
| With palladium 10% on activated carbon; zinc In water at 150℃; for 40h; Autoclave; | A 33 %Spectr. B 33 %Spectr. |
| With palladium 10% on activated carbon; zinc In water at 150℃; for 70h; Autoclave; | A 45 %Spectr. B 50 %Spectr. |

-
-
117273-45-7
5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinoline-2-carboxylic acid

-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| With quinoline; copper oxide-chromium oxide; hydrogen at 190℃; |
-
-
1026530-18-6
[(E)-8-Allyl-3,4-dihydro-2H-quinolin-1-ylimino]-acetic acid

-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| With potassium hydroxide; sulfuric acid 1.) ethanol, 120 deg C, 4 h, 2.) ethanol, reflux, 4 h; Yield given. Multistep reaction; |

-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| With pyridine; sulfuric acid; acetic anhydride 1.) ethanol, 3.5 h; Yield given. Multistep reaction; |
-
-
194231-71-5
1-allyl-7-bromo-1H-indole

-
A
-
16886-08-1
1-allylindole

-
B
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In toluene for 12h; Heating; Yield given. Yields of byproduct given; | |
| With 2,2'-azobis(isobutyronitrile); tri-n-butyl-tin hydride In toluene for 12h; Product distribution; Heating; different N-substituted-7-bromoindoles; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: tetrahydrofuran / 24 h / Ambient temperature 2: MgCl2 / 2-methoxy-ethanol; tetrahydrofuran / 6 h / 125 °C 3: 94 percent / aq. NaOH / ethanol / 2 h / Heating 4: 93 percent / copper chromite, quinoline / 3 h / 185 °C View Scheme | |
| Multi-step reaction with 2 steps 1: palladium 10% on activated carbon / 24 h / 150 °C / Autoclave 2: palladium 10% on activated carbon; zinc / water / 24 h / 150 °C / Autoclave View Scheme | |
| Multi-step reaction with 2 steps 1: palladium 10% on activated carbon / 24 h / 150 °C / Autoclave 2: palladium 10% on activated carbon; zinc / water / 70 h / 150 °C / Autoclave View Scheme |
-
-
124730-53-6
5,6-dihydro-4H-pyrrolo [3,2,1-ij]quinoline-1-carboxylic acid ethyl ester

-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 94 percent / aq. NaOH / ethanol / 2 h / Heating 2: 93 percent / copper chromite, quinoline / 3 h / 185 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydroxide; water / ethanol / 2 h / Reflux 2: copper chromite / quinoline / 2 h / 185 °C View Scheme |
-
-
152712-44-2
3-(3,4-dihydro-2H-quinolin-1-yl)-2-oxopropionic acid ethyl ester

-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: MgCl2 / 2-methoxy-ethanol; tetrahydrofuran / 6 h / 125 °C 2: 94 percent / aq. NaOH / ethanol / 2 h / Heating 3: 93 percent / copper chromite, quinoline / 3 h / 185 °C View Scheme | |
| Multi-step reaction with 3 steps 1: magnesium chloride / 2-methoxy-ethanol / 6 h / 125 °C / Reflux 2: sodium hydroxide; water / ethanol / 2 h / Reflux 3: copper chromite / quinoline / 2 h / 185 °C View Scheme |
-
-
92679-18-0
8-allyl-1,2,3,4-tetrahydroquinoline hydrochloride

-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 94.8 percent / CF3COOH / acetonitrile / 4 h 2: 88.6 percent / 1.8 N aq. sodium hypochlorite, 2.6 N aq. NaOH / ethanol / 1 h / 70 °C 3: 93 percent / 66percent aq. AcOH / 1 h / Ambient temperature 4: 1.) 10percent aq. H2SO4, 2.) Ac2O, pyridine / 1.) ethanol, 3.5 h View Scheme | |
| Multi-step reaction with 4 steps 1: 94.8 percent / CF3COOH / acetonitrile / 4 h 2: 88.6 percent / 1.8 N aq. sodium hypochlorite, 2.6 N aq. NaOH / ethanol / 1 h / 70 °C 3: 0.2percent aq. H2SO4 / ethanol / 2 h / Ambient temperature 4: 1.) aq. H2SO4, 2.) 30percent aq. KOH / 1.) ethanol, 120 deg C, 4 h, 2.) ethanol, reflux, 4 h View Scheme |
-
-
152771-24-9
8-allyl-1-carbamoyl-1,2,3,4-tetrahydroquinoline

-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 88.6 percent / 1.8 N aq. sodium hypochlorite, 2.6 N aq. NaOH / ethanol / 1 h / 70 °C 2: 93 percent / 66percent aq. AcOH / 1 h / Ambient temperature 3: 1.) 10percent aq. H2SO4, 2.) Ac2O, pyridine / 1.) ethanol, 3.5 h View Scheme | |
| Multi-step reaction with 3 steps 1: 88.6 percent / 1.8 N aq. sodium hypochlorite, 2.6 N aq. NaOH / ethanol / 1 h / 70 °C 2: 0.2percent aq. H2SO4 / ethanol / 2 h / Ambient temperature 3: 1.) aq. H2SO4, 2.) 30percent aq. KOH / 1.) ethanol, 120 deg C, 4 h, 2.) ethanol, reflux, 4 h View Scheme |
-
-
152771-25-0
8-allyl-1-amino-1,2,3,4-tetrahydroquinoline

-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 93 percent / 66percent aq. AcOH / 1 h / Ambient temperature 2: 1.) 10percent aq. H2SO4, 2.) Ac2O, pyridine / 1.) ethanol, 3.5 h View Scheme | |
| Multi-step reaction with 2 steps 1: 0.2percent aq. H2SO4 / ethanol / 2 h / Ambient temperature 2: 1.) aq. H2SO4, 2.) 30percent aq. KOH / 1.) ethanol, 120 deg C, 4 h, 2.) ethanol, reflux, 4 h View Scheme |

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; zinc In water at 150℃; for 24h; Autoclave; | 30 %Spectr. |
-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

-
-
25015-63-8
4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane

| Conditions | Yield |
|---|---|
| With benzylidene dichloride; C14H14N3P; N-ethyl-N,N-diisopropylamine In acetonitrile for 16h; regioselective reaction; | 98% |
-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| In 2,2,2-trifluoroethanol at 40℃; for 12h; Inert atmosphere; Schlenk technique; Sealed tube; | 97% |
-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| With water-d2; silver trifluoromethanesulfonate In chloroform-d1 at 90℃; for 18h; regioselective reaction; | 96% |
-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| With Diphenylphosphinic chloride In acetonitrile at 60℃; for 1h; Schlenk technique; Inert atmosphere; | 95% |
-
-
67-56-1
methanol

-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

-
-
79-37-8
oxalyl dichloride

-
-
124-41-4
sodium methylate

-
-
345264-02-0
(5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)oxoacetic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline; oxalyl dichloride In tetrahydrofuran at 0℃; for 2.5h; Stage #2: methanol; sodium methylate In tetrahydrofuran at -78℃; | 94% |
| Stage #1: 5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline; oxalyl dichloride In tetrahydrofuran; dichloromethane at 0℃; for 0.5h; Stage #2: methanol; sodium methylate In tetrahydrofuran; dichloromethane at -78 - 20℃; for 2h; | 87% |

-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

-
-
4290-72-6
5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinoline-1,2-dione

| Conditions | Yield |
|---|---|
| With oxygen; Rose Bengal lactone In water; N,N-dimethyl-formamide for 48h; Inert atmosphere; Irradiation; | 93% |

| Conditions | Yield |
|---|---|
| With copper diacetate; 4,4'-di-tert-butyl-2,2'-bipyridine at 90℃; for 24h; Schlenk technique; regioselective reaction; | 92% |
-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

-
-
28272-96-0
2-vinylbenzaldehyde

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; tetrakis(acetonitrile)palladium bistriflate In acetonitrile at 60℃; for 18h; Inert atmosphere; Schlenk technique; Sealed tube; regioselective reaction; | 91% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-Dichloropyrimidine With aluminum (III) chloride In 1,2-dimethoxyethane at 20℃; for 0.333333h; Stage #2: 5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline In 1,2-dimethoxyethane at 80℃; for 6h; | 90.1% |
| With iron(III) chloride In 1,2-dichloro-ethane at 60℃; | |
| Stage #1: 2,6-Dichloropyrimidine With aluminum (III) chloride In 1,2-dimethoxyethane at 20℃; for 0.333333h; Stage #2: 5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline In 1,2-dimethoxyethane at 80℃; for 6h; | |
| Stage #1: 2,6-Dichloropyrimidine With aluminum (III) chloride In 1,2-dimethoxyethane at 20℃; for 0.333333h; Stage #2: 5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline In 1,2-dimethoxyethane at 80℃; for 6h; |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; potassium iodide at 60℃; for 12h; | 89% |
| With dipotassium peroxodisulfate; potassium iodide In water; N,N-dimethyl-formamide at 60℃; for 12h; Green chemistry; | 89% |
-
-
637-69-4
4-Methoxystyrene

-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

-
-
118334-83-1
1,3-dioxoisoindolin-2-yl (3r,5r,7r)-adamantane-1- carboxylate

| Conditions | Yield |
|---|---|
| With indium(III) triflate; tris[2-phenylpyridinato-C2,N]iridium(III) at 30℃; for 24h; Schlenk technique; Inert atmosphere; Irradiation; | 88% |

-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; copper(I) triflate benzene complex; 2,6-bis[(4S,5R)-4,5-diphenyl-4,5-dihydro-1,3-oxazol-2-yl]pyridine In methanol at 0℃; for 24h; Schlenk technique; Inert atmosphere; enantioselective reaction; | 86% |
-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

-
-
124-41-4
sodium methylate

-
-
345264-02-0
(5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)oxoacetic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline; oxalyl dichloride In diethyl ether at 0℃; for 0.5 - 0.75h; Stage #2: sodium methylate In methanol; diethyl ether at -78 - 20℃; | 85% |
-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

-
-
79-37-8
oxalyl dichloride

-
-
124-41-4
sodium methylate

-
-
345264-02-0
(5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)oxoacetic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline; oxalyl dichloride In diethyl ether at 0℃; Stage #2: sodium methylate In methanol at -78 - 20℃; | 85% |
| Stage #1: 5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline; oxalyl dichloride In diethyl ether at 0℃; for 0.666667h; Stage #2: sodium methylate In methanol; diethyl ether at -78 - 24℃; for 2h; | 84% |

-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; phosphonic acid diethyl ester In acetonitrile at 85℃; for 3h; Sealed tube; | 85% |

| Conditions | Yield |
|---|---|
| With air In 2,2,2-trifluoroethanol; water at 120℃; for 12h; Sealed tube; | 85% |
-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

-
-
65094-22-6
diethyl (bromodifluoromethyl)phosphonate

| Conditions | Yield |
|---|---|
| Stage #1: 5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline With water; iodine; thiourea; potassium iodide In 1,4-dioxane at 50℃; Stage #2: With sodium hydroxide In 1,4-dioxane at 50℃; for 1h; Stage #3: diethyl (bromodifluoromethyl)phosphonate In 1,4-dioxane at 20℃; for 4h; | 80% |

| Conditions | Yield |
|---|---|
| With 5-ethyl-1,3,7,8-tetramethylalloxazinium triflate; iodine; oxygen In acetonitrile at 50℃; under 760.051 Torr; for 36h; Green chemistry; | 79% |
-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| With oxygen; palladium diacetate; acetic acid In dimethyl sulfoxide at 40℃; under 760.051 Torr; for 24h; regioselective reaction; | 76% |

| Conditions | Yield |
|---|---|
| With indium(III) triflate; silver carbonate at 120℃; for 24h; Schlenk technique; Inert atmosphere; Sealed tube; | 75% |


| Conditions | Yield |
|---|---|
| With 5-ethyl-1,3,7,8-tetramethylalloxazinium triflate; iodine; oxygen In acetonitrile at 50℃; under 760.051 Torr; for 36h; Green chemistry; | 75% |
-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| In acetonitrile at 90℃; for 6h; Inert atmosphere; | 73% |

| Conditions | Yield |
|---|---|
| With copper diacetate; 4,4'-di-tert-butyl-2,2'-bipyridine at 90℃; for 24h; Schlenk technique; regioselective reaction; | 73% |
-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In dichloromethane at 0℃; Inert atmosphere; Molecular sieve; chemoselective reaction; | 72% |

| Conditions | Yield |
|---|---|
| With palladium(II) trimethylacetate; silver carbonate In dimethyl sulfoxide; N,N-dimethyl-formamide at 80℃; for 19h; | 72% |
-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol; bis(trifluoromethanesulfonyl)amide at 100℃; for 6h; Sealed tube; | 72% |
-
-
5840-01-7
5,6-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline

-
-
24415-66-5
7-Chloro-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidine

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol; bis(trifluoromethanesulfonyl)amide at 100℃; for 6h; Sealed tube; | 72% |
| With 1,1,1,3',3',3'-hexafluoro-propanol; bis(trifluoromethanesulfonyl)amide at 100℃; for 6h; Microwave irradiation; Sealed tube; | 72% |
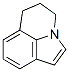
Lilolidine Chemical Properties
Molecule structure of Lilolidine (CAS NO.5840-01-7):
Molecular Weight: 157.2117 g/mol
Molecular Formula: C11H11N
Density: 1.166 g/cm3
Melting Point: 80-81°C
Boiling Point: 318.377 °C at 760 mmHg
Flash Point: 146.349 °C
Index of Refraction: 1.656
Molar Refractivity: 49.546 cm3
Molar Volume: 134.785 cm3
Polarizability: 19.641×10-24 cm3
Surface Tension: 44.838 dyne/cm
Enthalpy of Vaporization: 53.758 kJ/mol
Vapour Pressure: 0.001 mmHg at 25 °C
InChI: InChI=1/C11H11N/c1-3-9-5-2-7-12-8-6-10(4-1)11(9)12/h1,3-4,6,8H,2,5,7H2 Copy
InChIKey: QCCKSFHMARIKSK-UHFFFAOYAA
Product Categories of Lilolidine (CAS NO.5840-01-7): Pale White Powder
Lilolidine Specification
Lilolidine (CAS NO.5840-01-7) is also named as 2,3-dihydro-1H-pyrrolo[3,2,1-ij]quinoline ; 5,6-dihydro-4h-pyrrolo[3,2,1-ij]quinoline ; 1,7-Trimethyleneindole .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View