-
Name
2-Methyl-2-penten-4-one
- EINECS 205-502-5
- CAS No. 141-79-7
- Article Data383
- CAS DataBase
- Density 0.83 g/cm3
- Solubility 28 G/L (20 ºC)
- Melting Point -53 °C(lit.)
- Formula C6H10O
- Boiling Point 132.7 °C at 760 mmHg
- Molecular Weight 98.1448
- Flash Point 30.6 °C
- Transport Information UN 1229 3/PG 3
- Appearance clear light yellow liquid
- Safety 25
- Risk Codes 10-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 2,2-Dimethylvinylmethyl ketone;2-Methyl-2-penten-4-one;2-Methyl-4-oxo-2-pentene;4-Methyl-3-pentene-2-one;Isobutenyl methyl ketone;Isopropylideneacetone;Methyl 2,2-dimethylvinyl ketone;Methyl2-methyl-1-propenyl ketone;Methyl isobutenyl ketone;NSC 38717;
- PSA 17.07000
- LogP 1.54160
Synthetic route

| Conditions | Yield |
|---|---|
| With (Bu4N)2S2O8 In 1,2-dichloro-ethane for 1.5h; Heating; | 94.8% |
| With dihydrogen peroxide; tripropylammonium fluorochromate (VI) In acetone at 0 - 10℃; for 4h; | 92% |
| With perchloric acid; dihydrogen peroxide; potassium bromide; ammonium molybdate tetrahydrate In water at 20℃; for 1h; | 90% |
| With hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide In water; acetic acid at 20℃; for 12h; | 70% |
-
-
123-42-2
4-Hydroxy-4-methyl-2-pentanone

-
-
25290-14-6
4-Hydroxy-heptan-2-on

-
A
-
141-79-7
4-methyl-pent-3-en-2-one

-
B
-
5609-09-6, 69668-88-8, 1119-44-4
3-hepten-2-one

| Conditions | Yield |
|---|---|
| With sulfuric acid In water at 100 - 120℃; for 8h; | A 92.56% B 94.48% |


| Conditions | Yield |
|---|---|
| With hexaethylphosphoric triamide at 190℃; further reagent; | A 26.8% B 91.3% |

| Conditions | Yield |
|---|---|
| With 4-Hydroxy-4-methyl-2-pentanone at 190℃; | A 26.8% B 91.3% |
-
-
66647-68-5
4-bromo-4-methylpentan-2-one

-
-
141-79-7
4-methyl-pent-3-en-2-one

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In ethanol; water for 18h; Heating; | 88% |
| With triethylamine In water for 0.166667h; | 84% |

| Conditions | Yield |
|---|---|
| With tetrachlorosilane In chloroform for 5h; Heating; | 87% |
| With acid | |
| With SULFAMIDE at 170 - 175℃; |
-
-
65447-92-9
methyl-2 (methyl-2 propene-1 yl)-2 dithiolanne-1,3

-
-
141-79-7
4-methyl-pent-3-en-2-one

| Conditions | Yield |
|---|---|
| With hydrogen bromide; dihydrogen peroxide In acetonitrile at 25℃; for 0.166667h; | 87% |

| Conditions | Yield |
|---|---|
| With (π-C5H5)2Zr{OC(CH3)2H}2; benzaldehyde In toluene for 8h; Heating; | 86% |
| With pyridiniumchlorochromate on aluminumoxide at 20℃; for 14h; | 78% |
| With tert.-butylhydroperoxide; cobalt(II) ethyl phosphonate In decane; acetonitrile at 80℃; for 10h; | 76% |
-
-
50461-99-9
4-methyl-4-phenylsulfanyl-pentan-2-one

-
A
-
141-79-7
4-methyl-pent-3-en-2-one

-
B
-
1212-08-4
S-Phenyl benzenethiosulfonate

-
C
-
1208-20-4, 6930-77-4, 133670-27-6
S-phenyl benzenethiosulfinate

-
D
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| With sodium periodate; phosphate buffer In tetrahydrofuran for 5h; Product distribution; | A 85% B 44% C n/a D 43% |

-
-
67-64-1
acetone

-
A
-
141-79-7
4-methyl-pent-3-en-2-one

-
B
-
504-20-1
phorone

-
C
-
123-42-2
4-Hydroxy-4-methyl-2-pentanone

| Conditions | Yield |
|---|---|
| With titanium tetrachloride; NCNMe2 In benzene at 25℃; | A 82% B 2% C 8% |
| With sodium hydroxide In benzene at 40℃; Mechanism; Kinetics; benzyltriethylammonium chloride presence; | |
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In benzene at 40℃; reaction order, effect of concentration on the initial rate; | |
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In benzene at 40℃; Mechanism; effect concentrations, initial rate; | |
| With MgO/ZrO2 mixed oxides at 249.84℃; |

-
-
23652-85-9
(Z)-4-(methylamino)-3-penten-2-one

-
-
676-58-4
methylmagnesium chloride

-
-
141-79-7
4-methyl-pent-3-en-2-one

| Conditions | Yield |
|---|---|
| With cerium(III) chloride Mechanism; multistep reaction; other β-enamino ketones and Grignarg reagents; var. times; | 81% |
| With cerium(III) chloride 1.) THF, -78 deg C, 2 h, 2.) from -78 deg C to RT, 2.5 h; Yield given. Multistep reaction; |

-
-
141-79-7
4-methyl-pent-3-en-2-one

| Conditions | Yield |
|---|---|
| With dimethyl sulfoxide at 160℃; | 79% |

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate; sodium iodide In acetonitrile at -10℃; for 0.0166667h; | 78% |
| With sodium hydroxide; thiourea dioxide; tetrabutylammomium bromide In tetrahydrofuran for 0.2h; Ambient temperature; | 40% |
-
-
74491-15-9
4-methyl-4-benzylsulphinylpentan-2-one

-
A
-
150-60-7
dibenzyl disulphide

-
B
-
141-79-7
4-methyl-pent-3-en-2-one

-
C
-
16302-98-0
petivericin

-
D
-
16601-40-4
S-benzyl phenyl-methanethiosulfonate

| Conditions | Yield |
|---|---|
| With pyrrolidine In cyclohexane at 50℃; for 2h; Product distribution; further amines; | A 23% B 76% C 49% D 22% |


-
A
-
141-79-7
4-methyl-pent-3-en-2-one

-
-
88968-53-0
cis-{Ru(O2CMe)2(PMe3)4}

| Conditions | Yield |
|---|---|
| With CH3COOH In benzene-d6 inert gas; soln. was frozen (liquid nitrogen); exposed to vacuum; heated at 85°C for 2 h; not isolated; NMR; | A 74% B >99 |
-
-
41321-86-2
4-ethylsulfanyl-4-methyl-pentan-2-one

-
-
141-79-7
4-methyl-pent-3-en-2-one

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide In ethanol electrolysis; | 73% |
| With 3-chloro-benzenecarboperoxoic acid In chloroform 1.) ice-bath, 2.) r.t., 15 h; | 58% |
| With aluminum oxide; potassium sulfate; potassium hydrogensulfate; potassium peroxomonosulfate 1.) 6 h, room temperature 2.) ether, 180 min, room temperature; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With aluminium trichloride In dichloromethane | 73% |

| Conditions | Yield |
|---|---|
| In ethanol for 5h; Heating; | A 71% B 72% |


| Conditions | Yield |
|---|---|
| With formic acid In ethanol for 5h; Heating; | A 71% B 72% |
-
-
64-19-7
acetic acid

-
-
2632-88-4
ethyl N,N'-tetraethyldiamidophosphite

-
A
-
685-91-6
diethylacetamide

-
B
-
141-79-7
4-methyl-pent-3-en-2-one

-
C
-
109-89-7
diethylamine

-
D
-
6260-32-8
2-ethoxy-3-hydroxy-3,5,5-trimethyl-1,2-oxaphospholane-2-oxide

| Conditions | Yield |
|---|---|
| With 4-Hydroxy-4-methyl-2-pentanone | A 46.9% B 50% C 27.4% D 71.3% |

-
-
123-42-2
4-Hydroxy-4-methyl-2-pentanone

-
-
2632-88-4
ethyl N,N'-tetraethyldiamidophosphite

-
A
-
685-91-6
diethylacetamide

-
B
-
141-79-7
4-methyl-pent-3-en-2-one

-
C
-
109-89-7
diethylamine

-
D
-
6260-32-8
2-ethoxy-3-hydroxy-3,5,5-trimethyl-1,2-oxaphospholane-2-oxide

| Conditions | Yield |
|---|---|
| With acetic acid | A 46.9% B 50% C 27.4% D 71.3% |

-
-
2632-88-4
ethyl N,N'-tetraethyldiamidophosphite

-
A
-
685-91-6
diethylacetamide

-
B
-
141-79-7
4-methyl-pent-3-en-2-one

-
C
-
109-89-7
diethylamine

-
D
-
6260-32-8
2-ethoxy-3-hydroxy-3,5,5-trimethyl-1,2-oxaphospholane-2-oxide

| Conditions | Yield |
|---|---|
| With 4-Hydroxy-4-methyl-2-pentanone; acetic acid | A 46.9% B 50% C 27.4% D 71.3% |

-
-
123-35-3
7-methyl-3-methene-1,6-octadiene

-
-
16339-21-2
N-nitroso-β-methylaminoisobutyl methyl ketone

-
A
-
141-79-7
4-methyl-pent-3-en-2-one

| Conditions | Yield |
|---|---|
| With palladium(II) acetylacetonate; potassium hydroxide In water at 0 - 25℃; for 24.5h; | A 35% B 3% C 69% |

-
-
67-64-1
acetone

-
-
108-95-2
phenol

-
A
-
80-05-7
BPA

-
B
-
141-79-7
4-methyl-pent-3-en-2-one

-
C
-
837-08-1
2-[1-(4-hydroxyphenyl)-1-methylethyl]-phenol

| Conditions | Yield |
|---|---|
| beta zeolite acidic form at 120℃; under 760.051 Torr; for 12h; | A 63.2% B 0.01% C 11.43% |

-
-
4894-61-5
trans-but-2-enyl chloride

-
-
3102-33-8
trans-3-penten-2-one

-
A
-
141-79-7
4-methyl-pent-3-en-2-one

-
-
112344-59-9
threo-3,4-dimethyl-1,5-heptadien-4-ol

| Conditions | Yield |
|---|---|
| With iodine; magnesium; ethylene dibromide In diethyl ether for 12h; Ambient temperature; Title compound not separated from byproducts; | A 40% B 60% |

-
-
100-99-2
triisobutylaluminum

-
-
75-36-5
acetyl chloride

-
A
-
141-79-7
4-methyl-pent-3-en-2-one

-
B
-
14575-13-4
4-chloro-4-methyl-2-pentanone

-
C
-
108-10-1
4-methyl-2-pentanone

| Conditions | Yield |
|---|---|
| With aluminium trichloride In dichloromethane for 1h; Product distribution; Ambient temperature; other reaction time and temperature; other molecular proportion.; | A 15% B 30% C 55% |
| With aluminium trichloride In dichloromethane for 1h; Ambient temperature; | A 55% B 30% C 15% |

-
-
110-87-2
3,4-dihydro-2H-pyran

-
-
123-42-2
4-Hydroxy-4-methyl-2-pentanone

-
A
-
57283-21-3
4-(tetrahydropyranyl-2-oxy)-4-methyl-2-pentanone

-
B
-
141-79-7
4-methyl-pent-3-en-2-one

| Conditions | Yield |
|---|---|
| With lithium hexafluorophosphate In hexane at 0℃; for 3h; | A 50% B n/a |


| Conditions | Yield |
|---|---|
| 48% | |
| With aluminum oxide for 192h; Ambient temperature; | 20% |
| Amberlite IR-120 at 60℃; for 24h; Product distribution; various quantity of catalyst, various temperatures and reaction times, also with Nafion-H; | 19.8% |
-
-
67-64-1
acetone

-
A
-
108-11-2
Methyl isobutyl carbinol

-
B
-
141-79-7
4-methyl-pent-3-en-2-one

-
C
-
108-10-1
4-methyl-2-pentanone

-
D
-
67-63-0
isopropyl alcohol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal; Nafion-H at 100℃; under 2585.7 Torr; for 24h; Further byproducts given; | A 3% B 3% C 48% D 37% |
| With hydrogen; palladium on activated charcoal; Nafion-H at 100℃; under 2585.7 Torr; for 24h; Product distribution; various pressures, temperatures and reaction times, also with Amberlite IR-120/ Pd/C; | A 3% B 3% C 48% D 37% |
| With hydrogen; palladium on activated charcoal; Amberlite IR-120 at 100℃; under 2585.7 Torr; for 24h; Further byproducts given; | A 1.2% B 3% C 40% D 42% |


| Conditions | Yield |
|---|---|
| With (Bu4N)2S2O8 In 1,2-dichloro-ethane for 1.5h; Heating; | 94.8% |
| With dihydrogen peroxide; tripropylammonium fluorochromate (VI) In acetone at 0 - 10℃; for 4h; | 92% |
| With perchloric acid; dihydrogen peroxide; potassium bromide; ammonium molybdate tetrahydrate In water at 20℃; for 1h; | 90% |
| With hexaammonium heptamolybdate tetrahydrate; dihydrogen peroxide In water; acetic acid at 20℃; for 12h; | 70% |
-
-
123-42-2
4-Hydroxy-4-methyl-2-pentanone

-
-
25290-14-6
4-Hydroxy-heptan-2-on

-
A
-
141-79-7
4-methyl-pent-3-en-2-one

-
B
-
5609-09-6, 69668-88-8, 1119-44-4
3-hepten-2-one

| Conditions | Yield |
|---|---|
| With sulfuric acid In water at 100 - 120℃; for 8h; | A 92.56% B 94.48% |


| Conditions | Yield |
|---|---|
| With hexaethylphosphoric triamide at 190℃; further reagent; | A 26.8% B 91.3% |

| Conditions | Yield |
|---|---|
| With 4-Hydroxy-4-methyl-2-pentanone at 190℃; | A 26.8% B 91.3% |
-
-
66647-68-5
4-bromo-4-methylpentan-2-one

-
-
141-79-7
4-methyl-pent-3-en-2-one

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In ethanol; water for 18h; Heating; | 88% |
| With triethylamine In water for 0.166667h; | 84% |

| Conditions | Yield |
|---|---|
| With tetrachlorosilane In chloroform for 5h; Heating; | 87% |
| With acid | |
| With SULFAMIDE at 170 - 175℃; |
-
-
65447-92-9
methyl-2 (methyl-2 propene-1 yl)-2 dithiolanne-1,3

-
-
141-79-7
4-methyl-pent-3-en-2-one

| Conditions | Yield |
|---|---|
| With hydrogen bromide; dihydrogen peroxide In acetonitrile at 25℃; for 0.166667h; | 87% |

| Conditions | Yield |
|---|---|
| With (π-C5H5)2Zr{OC(CH3)2H}2; benzaldehyde In toluene for 8h; Heating; | 86% |
| With pyridiniumchlorochromate on aluminumoxide at 20℃; for 14h; | 78% |
| With tert.-butylhydroperoxide; cobalt(II) ethyl phosphonate In decane; acetonitrile at 80℃; for 10h; | 76% |
-
-
50461-99-9
4-methyl-4-phenylsulfanyl-pentan-2-one

-
A
-
141-79-7
4-methyl-pent-3-en-2-one

-
B
-
1212-08-4
S-Phenyl benzenethiosulfonate

-
C
-
1208-20-4, 6930-77-4, 133670-27-6
S-phenyl benzenethiosulfinate

-
D
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| With sodium periodate; phosphate buffer In tetrahydrofuran for 5h; Product distribution; | A 85% B 44% C n/a D 43% |

-
-
67-64-1
acetone

-
A
-
141-79-7
4-methyl-pent-3-en-2-one

-
B
-
504-20-1
phorone

-
C
-
123-42-2
4-Hydroxy-4-methyl-2-pentanone

| Conditions | Yield |
|---|---|
| With titanium tetrachloride; NCNMe2 In benzene at 25℃; | A 82% B 2% C 8% |
| With sodium hydroxide In benzene at 40℃; Mechanism; Kinetics; benzyltriethylammonium chloride presence; | |
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In benzene at 40℃; reaction order, effect of concentration on the initial rate; | |
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In benzene at 40℃; Mechanism; effect concentrations, initial rate; | |
| With MgO/ZrO2 mixed oxides at 249.84℃; |

-
-
23652-85-9
(Z)-4-(methylamino)-3-penten-2-one

-
-
676-58-4
methylmagnesium chloride

-
-
141-79-7
4-methyl-pent-3-en-2-one

| Conditions | Yield |
|---|---|
| With cerium(III) chloride Mechanism; multistep reaction; other β-enamino ketones and Grignarg reagents; var. times; | 81% |
| With cerium(III) chloride 1.) THF, -78 deg C, 2 h, 2.) from -78 deg C to RT, 2.5 h; Yield given. Multistep reaction; |

-
-
141-79-7
4-methyl-pent-3-en-2-one

| Conditions | Yield |
|---|---|
| With dimethyl sulfoxide at 160℃; | 79% |

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate; sodium iodide In acetonitrile at -10℃; for 0.0166667h; | 78% |
| With sodium hydroxide; thiourea dioxide; tetrabutylammomium bromide In tetrahydrofuran for 0.2h; Ambient temperature; | 40% |
-
-
74491-15-9
4-methyl-4-benzylsulphinylpentan-2-one

-
A
-
150-60-7
dibenzyl disulphide

-
B
-
141-79-7
4-methyl-pent-3-en-2-one

-
C
-
16302-98-0
petivericin

-
D
-
16601-40-4
S-benzyl phenyl-methanethiosulfonate

| Conditions | Yield |
|---|---|
| With pyrrolidine In cyclohexane at 50℃; for 2h; Product distribution; further amines; | A 23% B 76% C 49% D 22% |


-
A
-
141-79-7
4-methyl-pent-3-en-2-one

-
-
88968-53-0
cis-{Ru(O2CMe)2(PMe3)4}

| Conditions | Yield |
|---|---|
| With CH3COOH In benzene-d6 inert gas; soln. was frozen (liquid nitrogen); exposed to vacuum; heated at 85°C for 2 h; not isolated; NMR; | A 74% B >99 |
-
-
41321-86-2
4-ethylsulfanyl-4-methyl-pentan-2-one

-
-
141-79-7
4-methyl-pent-3-en-2-one

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide In ethanol electrolysis; | 73% |
| With 3-chloro-benzenecarboperoxoic acid In chloroform 1.) ice-bath, 2.) r.t., 15 h; | 58% |
| With aluminum oxide; potassium sulfate; potassium hydrogensulfate; potassium peroxomonosulfate 1.) 6 h, room temperature 2.) ether, 180 min, room temperature; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With aluminium trichloride In dichloromethane | 73% |

| Conditions | Yield |
|---|---|
| In ethanol for 5h; Heating; | A 71% B 72% |


| Conditions | Yield |
|---|---|
| With formic acid In ethanol for 5h; Heating; | A 71% B 72% |
-
-
64-19-7
acetic acid

-
-
2632-88-4
ethyl N,N'-tetraethyldiamidophosphite

-
A
-
685-91-6
diethylacetamide

-
B
-
141-79-7
4-methyl-pent-3-en-2-one

-
C
-
109-89-7
diethylamine

-
D
-
6260-32-8
2-ethoxy-3-hydroxy-3,5,5-trimethyl-1,2-oxaphospholane-2-oxide

| Conditions | Yield |
|---|---|
| With 4-Hydroxy-4-methyl-2-pentanone | A 46.9% B 50% C 27.4% D 71.3% |

-
-
123-42-2
4-Hydroxy-4-methyl-2-pentanone

-
-
2632-88-4
ethyl N,N'-tetraethyldiamidophosphite

-
A
-
685-91-6
diethylacetamide

-
B
-
141-79-7
4-methyl-pent-3-en-2-one

-
C
-
109-89-7
diethylamine

-
D
-
6260-32-8
2-ethoxy-3-hydroxy-3,5,5-trimethyl-1,2-oxaphospholane-2-oxide

| Conditions | Yield |
|---|---|
| With acetic acid | A 46.9% B 50% C 27.4% D 71.3% |

-
-
2632-88-4
ethyl N,N'-tetraethyldiamidophosphite

-
A
-
685-91-6
diethylacetamide

-
B
-
141-79-7
4-methyl-pent-3-en-2-one

-
C
-
109-89-7
diethylamine

-
D
-
6260-32-8
2-ethoxy-3-hydroxy-3,5,5-trimethyl-1,2-oxaphospholane-2-oxide

| Conditions | Yield |
|---|---|
| With 4-Hydroxy-4-methyl-2-pentanone; acetic acid | A 46.9% B 50% C 27.4% D 71.3% |

-
-
123-35-3
7-methyl-3-methene-1,6-octadiene

-
-
16339-21-2
N-nitroso-β-methylaminoisobutyl methyl ketone

-
A
-
141-79-7
4-methyl-pent-3-en-2-one

| Conditions | Yield |
|---|---|
| With palladium(II) acetylacetonate; potassium hydroxide In water at 0 - 25℃; for 24.5h; | A 35% B 3% C 69% |

-
-
67-64-1
acetone

-
-
108-95-2
phenol

-
A
-
80-05-7
BPA

-
B
-
141-79-7
4-methyl-pent-3-en-2-one

-
C
-
837-08-1
2-[1-(4-hydroxyphenyl)-1-methylethyl]-phenol

| Conditions | Yield |
|---|---|
| beta zeolite acidic form at 120℃; under 760.051 Torr; for 12h; | A 63.2% B 0.01% C 11.43% |

-
-
4894-61-5
trans-but-2-enyl chloride

-
-
3102-33-8
trans-3-penten-2-one

-
A
-
141-79-7
4-methyl-pent-3-en-2-one

-
-
112344-59-9
threo-3,4-dimethyl-1,5-heptadien-4-ol

| Conditions | Yield |
|---|---|
| With iodine; magnesium; ethylene dibromide In diethyl ether for 12h; Ambient temperature; Title compound not separated from byproducts; | A 40% B 60% |

-
-
100-99-2
triisobutylaluminum

-
-
75-36-5
acetyl chloride

-
A
-
141-79-7
4-methyl-pent-3-en-2-one

-
B
-
14575-13-4
4-chloro-4-methyl-2-pentanone

-
C
-
108-10-1
4-methyl-2-pentanone

| Conditions | Yield |
|---|---|
| With aluminium trichloride In dichloromethane for 1h; Product distribution; Ambient temperature; other reaction time and temperature; other molecular proportion.; | A 15% B 30% C 55% |
| With aluminium trichloride In dichloromethane for 1h; Ambient temperature; | A 55% B 30% C 15% |

-
-
110-87-2
3,4-dihydro-2H-pyran

-
-
123-42-2
4-Hydroxy-4-methyl-2-pentanone

-
A
-
57283-21-3
4-(tetrahydropyranyl-2-oxy)-4-methyl-2-pentanone

-
B
-
141-79-7
4-methyl-pent-3-en-2-one

| Conditions | Yield |
|---|---|
| With lithium hexafluorophosphate In hexane at 0℃; for 3h; | A 50% B n/a |


| Conditions | Yield |
|---|---|
| 48% | |
| With aluminum oxide for 192h; Ambient temperature; | 20% |
| Amberlite IR-120 at 60℃; for 24h; Product distribution; various quantity of catalyst, various temperatures and reaction times, also with Nafion-H; | 19.8% |
-
-
67-64-1
acetone

-
A
-
108-11-2
Methyl isobutyl carbinol

-
B
-
141-79-7
4-methyl-pent-3-en-2-one

-
C
-
108-10-1
4-methyl-2-pentanone

-
D
-
67-63-0
isopropyl alcohol

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal; Nafion-H at 100℃; under 2585.7 Torr; for 24h; Further byproducts given; | A 3% B 3% C 48% D 37% |
| With hydrogen; palladium on activated charcoal; Nafion-H at 100℃; under 2585.7 Torr; for 24h; Product distribution; various pressures, temperatures and reaction times, also with Amberlite IR-120/ Pd/C; | A 3% B 3% C 48% D 37% |
| With hydrogen; palladium on activated charcoal; Amberlite IR-120 at 100℃; under 2585.7 Torr; for 24h; Further byproducts given; | A 1.2% B 3% C 40% D 42% |

-
-
141-79-7
4-methyl-pent-3-en-2-one

-
-
1826-67-1
vinyl magnesium bromide

-
-
38552-68-0
3,5-dimethyl-hexa-1,4-dien-3-ol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran | 100% |

| Conditions | Yield |
|---|---|
| With potassium Sodium; polyethylene oxide In tetrahydrofuran at 0℃; 2.5 ethylene oxide units/M(+); | 100% |
| With formic acid; C18H24ClIrN3 In water at 80℃; for 4h; Schlenk technique; Inert atmosphere; | 80% |
| With nickel at 110 - 165℃; under 73550.8 - 147102 Torr; Hydrogenation; |
Mesityl oxide Consensus Reports
Mesityl oxide Standards and Recommendations
ACGIH TLV: TWA 15 ppm; STEL 25 ppm
DFG MAK: 25 ppm (100 mg/m3)
NIOSH REL: (Ketones) TWA 40 mg/m3
DOT Classification: 3; Label: Flammable Liquid
Mesityl oxide Analytical Methods
Mesityl oxide Specification
The Mesityl oxide, with the CAS registry number 141-79-7 and EINECS registry number 205-502-5, has the systhematic name of 4-methylpent-3-en-2-one. It is a is a α,β-Unsaturated ketone with the molecular formula of C6H10O. It is a kind of clear light yellow liquid, and belongs to the product category of Acetone series. What's more, it is usually used as mid-boiling point strong solvent.
The physical properties of Mesityl oxide are as followings: (1)ACD/LogP: 1.12; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.11; (4)ACD/LogD (pH 7.4): 1.11; (5)ACD/BCF (pH 5.5): 4.14; (6)ACD/BCF (pH 7.4): 4.14; (7)ACD/KOC (pH 5.5): 96.26; (8)ACD/KOC (pH 7.4): 96.26; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 17.07 Å2; (13)Index of Refraction: 1.418; (14)Molar Refractivity: 29.77 cm3; (15)Molar Volume: 118.1 cm3; (16)Polarizability: 11.8×10-24cm3; (17)Surface Tension: 23.2 dyne/cm; (18)Density: 0.83 g/cm3; (19)Flash Point: 30.6 °C; (20)Enthalpy of Vaporization: 37.02 kJ/mol; (21)Boiling Point: 132.7 °C at 760 mmHg; (22)Vapour Pressure: 8.76 mmHg at 25°C.
Synthesis: It can be prepared by the aldol condensation of acetone to give diacetone alcohol, which readily dehydrates to give this compound.
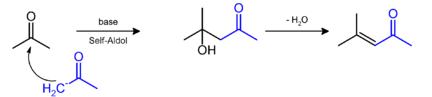
Uses of Mesityl oxide: It can be used as a solvent and in the production of methyl isobutyl ketone by hydrogenation:

You should be cautious while dealing with this chemical. It is a kind of flammble chemical which is also harmful by inhalation, in contact with skin and if swallowed. Therefore, you had better avoid contact with eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(\C=C(/C)C)C
(2)InChI: InChI=1/C6H10O/c1-5(2)4-6(3)7/h4H,1-3H3
(3)InChIKey: SHOJXDKTYKFBRD-UHFFFAOYAQ
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| frog | LDLo | subcutaneous | 1400mg/kg (1400mg/kg) | Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 56, Pg. 346, 1906. | |
| guinea pig | LCLo | inhalation | 500ppm/8H (500ppm) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Journal of Industrial Hygiene and Toxicology. Vol. 24, Pg. 46, 1942. |
| human | TCLo | inhalation | 25ppm (25ppm) | SENSE ORGANS AND SPECIAL SENSES: CONJUNCTIVE IRRITATION: EYE | Journal of Industrial Hygiene and Toxicology. Vol. 28, Pg. 262, 1946. |
| mammal (species unspecified) | LD50 | oral | 710mg/kg (710mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 39(4), Pg. 86, 1974. | |
| mouse | LC50 | inhalation | 10gm/m3/2H (10000mg/m3) | "Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure," Izmerov, N.F., et al., Moscow, Centre of International Projects, GKNT, 1982Vol. -, Pg. 80, 1982. | |
| mouse | LD50 | intraperitoneal | 354mg/kg (354mg/kg) | Shell Chemical Company. Unpublished Report. Vol. -, Pg. 6, 1961. | |
| mouse | LD50 | oral | 710mg/kg (710mg/kg) | "Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure," Izmerov, N.F., et al., Moscow, Centre of International Projects, GKNT, 1982Vol. -, Pg. 80, 1982. | |
| rabbit | LD50 | oral | 1gm/kg (1000mg/kg) | "Documentation of the Threshold Limit Values and Biological Exposure Indices," 5th ed., Cincinnati, OH, American Conference of Governmental Industrial Hygienists, Inc., 1986Vol. 6, Pg. 896, 1991. | |
| rabbit | LD50 | skin | 5150mg/kg (5150mg/kg) | Raw Material Data Handbook, Vol.1: Organic Solvents, 1974. Vol. 1, Pg. 71, 1974. | |
| rabbit | LDLo | subcutaneous | 840mg/kg (840mg/kg) | Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 56, Pg. 346, 1906. | |
| rat | LC50 | inhalation | 9gm/m3/4H (9000mg/m3) | "Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure," Izmerov, N.F., et al., Moscow, Centre of International Projects, GKNT, 1982Vol. -, Pg. 80, 1982. | |
| rat | LD50 | oral | 1120mg/kg (1120mg/kg) | "Documentation of the Threshold Limit Values and Biological Exposure Indices," 5th ed., Cincinnati, OH, American Conference of Governmental Industrial Hygienists, Inc., 1986Vol. 6, Pg. 896, 1991. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View