-
Name
DIMETHYLMALONONITRILE
- EINECS
- CAS No. 7321-55-3
- Article Data11
- CAS DataBase
- Density 0.961 g/cm3
- Solubility
- Melting Point 31-33 °C(lit.)
- Formula C5H6N2
- Boiling Point 169.5 °C at 760 mmHg
- Molecular Weight 94.116
- Flash Point 77.8 °C
- Transport Information
- Appearance
- Safety 16-26-36/37/39-45
- Risk Codes 11-23/24/25-36/37/38
-
Molecular Structure
-
Hazard Symbols
 F;
F;  T
T
- Synonyms Malononitrile,dimethyl- (6CI,7CI,8CI);Propanedinitrile, dimethyl- (9CI);2,2-Dicyanopropane;Dimethylmalononitrile;Dimethylpropanedinitrile;NSC 86109;2,2-Dicyanopropane;Dimethylmalononitrile;Malononitrile, dimethyl-;propanedinitrile, 2,2-dimethyl-;
- PSA 47.58000
- LogP 1.05976
Synthetic route

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; tetrabutylammomium bromide for 2h; Ambient temperature; | 76% |
| With potassium carbonate In acetonitrile at 20℃; for 16h; Inert atmosphere; | 52% |
| at 100℃; aus Natriummalonnitril; | |
| With sodium hydride In dimethyl sulfoxide |

| Conditions | Yield |
|---|---|
| Stage #1: malononitrile With lithium hydroxide In tetrahydrofuran at 5 - 20℃; for 3h; Autoclave; Stage #2: methyl bromide In tetrahydrofuran at 0 - 20℃; for 8h; Autoclave; | 46.5% |

| Conditions | Yield |
|---|---|
| With phosphorus pentaoxide at 170℃; |

| Conditions | Yield |
|---|---|
| With triphenylphosphine |

| Conditions | Yield |
|---|---|
| at -10℃; aus Silbermalonnitril nach Beendigung der ersten heftigen Reaktion bei 50grad; |

-
-
19295-57-9
3-hydroxy-2,2-dimethylpropionitrile

-
-
7321-55-3
2,2-dimethylmalononitrile

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: oxalyl dichloride / dimethyl sulfoxide; dichloromethane / 0.42 h / -78 °C 1.2: -78 - 25 °C 2.1: methanol; water; hydrogenchloride / 1 h / 55 °C 3.1: tetrabutyl ammonium fluoride / dimethyl sulfoxide / 0.25 h / 25 °C View Scheme |
-
-
19295-56-8
2,2-dimethyl-3-oxopropanenitrile

-
-
7321-55-3
2,2-dimethylmalononitrile

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: methanol; water; hydrogenchloride / 1 h / 55 °C 2: tetrabutyl ammonium fluoride / dimethyl sulfoxide / 0.25 h / 25 °C View Scheme |
-
-
1401327-14-7
2’-O-(2-cyano-2,2-dimethylethanimine-N-oxymethyl)uridine

-
A
-
50-00-0
formaldehyd

-
B
-
7321-55-3
2,2-dimethylmalononitrile

-
C
-
58-96-8
uridine

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In dimethyl sulfoxide at 25℃; for 0.25h; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: potassium carbonate; water; hydroxylamine hydrochloride / ethanol / 48 h / 70 °C / Cooling with ice 2.1: trifluoroacetic anhydride / -15 °C / Reflux 3.1: methanol / 16 h / 25 °C 4.1: oxalyl dichloride / dimethyl sulfoxide; dichloromethane / 0.42 h / -78 °C 4.2: -78 - 25 °C 5.1: methanol; water; hydrogenchloride / 1 h / 55 °C 6.1: tetrabutyl ammonium fluoride / dimethyl sulfoxide / 0.25 h / 25 °C View Scheme |
-
-
36559-87-2
3-hydroxy-2,2-dimethylpropionaldoxime

-
-
7321-55-3
2,2-dimethylmalononitrile

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: trifluoroacetic anhydride / -15 °C / Reflux 2.1: methanol / 16 h / 25 °C 3.1: oxalyl dichloride / dimethyl sulfoxide; dichloromethane / 0.42 h / -78 °C 3.2: -78 - 25 °C 4.1: methanol; water; hydrogenchloride / 1 h / 55 °C 5.1: tetrabutyl ammonium fluoride / dimethyl sulfoxide / 0.25 h / 25 °C View Scheme |

-
-
7321-55-3
2,2-dimethylmalononitrile

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: methanol / 16 h / 25 °C 2.1: oxalyl dichloride / dimethyl sulfoxide; dichloromethane / 0.42 h / -78 °C 2.2: -78 - 25 °C 3.1: methanol; water; hydrogenchloride / 1 h / 55 °C 4.1: tetrabutyl ammonium fluoride / dimethyl sulfoxide / 0.25 h / 25 °C View Scheme |
-
-
24423-87-8
3,4-dihydro-isoquinoline 2-oxide

-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
121994-34-1
2-(5,9b-Dihydro-4H-3-oxa-1,3a-diaza-cyclopenta[a]naphthalen-2-yl)-2-methyl-propionitrile

| Conditions | Yield |
|---|---|
| In toluene at 100℃; for 1h; | 100% |
| In toluene at 100℃; Rate constant; two other solvents; |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
106544-20-1
2-oxo-3-(ethoxycarbonyl)-3,4-dihydro-β-carboline

-
-
106544-23-4
2-(1-cyano-1-methylethyl)-5-(ethoxycarbonyl)-4,5,6,11b-tetrahydro-Δ4-1,2,4-oxadiazolino<3,2-a>-β-carboline

| Conditions | Yield |
|---|---|
| In toluene at 80℃; for 1h; | 100% |
| In toluene at 80℃; for 1.5h; | 99% |
| In 2-methoxy-ethanol at 100℃; Rate constant; |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
3182-95-4
L-Phenylalaninol

-
-
176706-98-2
(4S,4S')-(-)-2,2'-(1-methylethylidene)bis[4,5-dihydro-4-(phenylmethyl)oxazole]

| Conditions | Yield |
|---|---|
| With zinc trifluoromethanesulfonate In toluene for 48h; Heating; | 100% |
| With zinc(II) chloride In chlorobenzene for 24h; Inert atmosphere; Reflux; | 75% |
| With zinc(II) chloride In chlorobenzene for 72h; Reflux; | 24% |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
136030-00-7
(1R,2S)-1-Amino-2-indanol

| Conditions | Yield |
|---|---|
| With zinc trifluoromethanesulfonate In toluene for 48h; Heating; | 100% |
| Stage #1: 2,2-dimethylmalononitrile With zinc trifluoromethanesulfonate In toluene for 0.0833333h; Inert atmosphere; Stage #2: (1R,2S)-1-Amino-2-indanol In toluene for 24h; Reflux; Inert atmosphere; | 71% |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
5720-07-0
4-methoxyphenylboronic acid

-
-
118362-74-6
3-(4-methoxyphenyl)-2,2-dimethyl-3-oxopropanenitrile

| Conditions | Yield |
|---|---|
| Stage #1: 2,2-dimethylmalononitrile; 4-methoxyphenylboronic acid With [Rh(OH)(cod)]2; N,N,N',N'-tetramethyl-1,8-diaminonaphthalene In 1,4-dioxane at 80℃; for 6h; Inert atmosphere; Stage #2: With hydrogenchloride; water In 1,4-dioxane; tert-butyl methyl ether Inert atmosphere; | 99% |
| With 4,4'-dimethyl-2,2'-bipyridines; palladium(II) acetylacetonate; toluene-4-sulfonic acid In water; toluene at 80℃; for 24h; Schlenk technique; | 75% |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
100-39-0
benzyl bromide

-
-
140-29-4
phenylacetonitrile

-
-
861356-28-7
2-benzyl-2-phenylmalononitrile

| Conditions | Yield |
|---|---|
| Stage #1: phenylacetonitrile With methylmagnesium bromide; lithium chloride In tetrahydrofuran; diethyl ether at 20℃; for 0.5h; Inert atmosphere; Stage #2: 2,2-dimethylmalononitrile In tetrahydrofuran; diethyl ether at 80℃; for 6h; Inert atmosphere; Stage #3: benzyl bromide In tetrahydrofuran; diethyl ether; N,N-dimethyl-formamide at 80℃; for 16h; Inert atmosphere; | 99% |


| Conditions | Yield |
|---|---|
| Stage #1: pentanonitrile With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -78℃; for 1h; Inert atmosphere; Stage #2: 2,2-dimethylmalononitrile In tetrahydrofuran; hexane at 20 - 80℃; for 6h; Inert atmosphere; Stage #3: benzyl bromide In tetrahydrofuran; hexane; N,N-dimethyl-formamide at 80℃; for 16h; Inert atmosphere; | 98% |

-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
5720-07-0
4-methoxyphenylboronic acid

-
-
874-90-8
4-methoxybenzonitrile

| Conditions | Yield |
|---|---|
| With chloro(1,5-cyclooctadiene)rhodium(I) dimer; caesium carbonate In 1,4-dioxane at 100℃; for 6h; Inert atmosphere; | 97% |
-
-
943599-11-9
(2S)-2-amino-3-(diphenylphosphoryl)-propan-1-ol

-
-
7321-55-3
2,2-dimethylmalononitrile

| Conditions | Yield |
|---|---|
| Stage #1: (2S)-2-amino-3-(diphenylphosphoryl)-propan-1-ol; 2,2-dimethylmalononitrile With zinc(II) chloride In chlorobenzene for 24h; Heating; Stage #2: With ethylenediamine In water; chlorobenzene for 1h; Further stages.; | 96% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 23℃; for 0.5h; | 96% |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
52287-51-1
6-bromo-1,4-benzodioxane

-
-
19102-07-9
2,3-dihydrobenzo[b][1,4]dioxine-6-carbonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 6-bromo-1,4-benzodioxane With magnesium; lithium chloride In tetrahydrofuran at 23℃; Stage #2: 2,2-dimethylmalononitrile In tetrahydrofuran at 0 - 23℃; for 0.5h; | 96% |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
63104-44-9
dimethyl 2,2-di(prop-2-ynyl)malonate

| Conditions | Yield |
|---|---|
| With tetraethylammonium chloride; tris(acetonitrile)pentamethylcyclopentadienylruthenium(II) hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 0.16h; | 95% |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
98-80-6
phenylboronic acid

-
-
7391-73-3
2,2-dimethyl-3-oxo-3-phenylpropanenitrile

| Conditions | Yield |
|---|---|
| Stage #1: 2,2-dimethylmalononitrile; phenylboronic acid With [Rh(OH)(cod)]2; N,N,N',N'-tetramethyl-1,8-diaminonaphthalene In 1,4-dioxane at 80℃; for 4h; Inert atmosphere; Stage #2: With hydrogenchloride; water In 1,4-dioxane; tert-butyl methyl ether Reagent/catalyst; Inert atmosphere; | 95% |
| With 4,4'-dimethyl-2,2'-bipyridines; palladium(II) acetylacetonate; toluene-4-sulfonic acid In water; toluene at 80℃; for 24h; Schlenk technique; | 95% |
| With 4,4'-dimethyl-2,2'-bipyridines; water; palladium(II) acetylacetonate; benzenesulfonic acid In toluene at 80℃; for 24h; | 84% |
| With bis(norbornadiene)rhodium(l)tetrafluoroborate; potassium carbonate In 1,4-dioxane at 80℃; for 24h; Reagent/catalyst; Inert atmosphere; | 45 %Chromat. |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
5720-05-8
4-methylphenylboronic acid

-
-
118362-75-7
2,2-dimethyl-3-oxo-3-(p-tolyl)propanenitrile

| Conditions | Yield |
|---|---|
| Stage #1: 2,2-dimethylmalononitrile; 4-methylphenylboronic acid With [Rh(OH)(cod)]2; N,N,N',N'-tetramethyl-1,8-diaminonaphthalene In 1,4-dioxane at 80℃; for 6h; Inert atmosphere; Stage #2: With hydrogenchloride; water In 1,4-dioxane; tert-butyl methyl ether Inert atmosphere; | 95% |
| With 4,4'-dimethyl-2,2'-bipyridines; palladium(II) acetylacetonate; toluene-4-sulfonic acid In water; toluene at 80℃; for 24h; Schlenk technique; | 90% |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
140-29-4
phenylacetonitrile

-
-
74-88-4
methyl iodide

-
-
86164-70-7
2-methyl-2-phenylmalononitrile

| Conditions | Yield |
|---|---|
| Stage #1: phenylacetonitrile With methylmagnesium bromide; lithium chloride In tetrahydrofuran; diethyl ether at 20℃; for 0.5h; Inert atmosphere; Stage #2: 2,2-dimethylmalononitrile In tetrahydrofuran; diethyl ether at 80℃; for 6h; Inert atmosphere; Stage #3: methyl iodide In tetrahydrofuran; diethyl ether; N,N-dimethyl-formamide at 80℃; for 16h; Inert atmosphere; | 95% |

-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
22426-30-8
2-cyano-2-methylpropanoic acid

| Conditions | Yield |
|---|---|
| enzyme from Synechocystis sp. PCC 6803 In phosphate buffer at 30℃; for 12h; | 94% |
| With Bradyrhizobium japonicum strain USDA110 nitrilase bll6402 In phosphate buffer at 30℃; for 24h; pH=7.2; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: 6-iodo-chroman With isopropylmagnesium chloride In tetrahydrofuran at 0℃; for 0.5h; Stage #2: 2,2-dimethylmalononitrile In tetrahydrofuran at 0 - 23℃; for 0.5h; | 94% |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
5720-05-8
4-methylphenylboronic acid

-
-
104-85-8
para-methylbenzonitrile

| Conditions | Yield |
|---|---|
| With chloro(1,5-cyclooctadiene)rhodium(I) dimer; caesium carbonate In 1,4-dioxane at 100℃; for 6h; Inert atmosphere; | 94% |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
7480-35-5, 13286-59-4, 74165-73-4, 126456-43-7, 136030-00-7, 140632-19-5, 140632-20-8
(1S,2R)-1-amino-2-indanol

| Conditions | Yield |
|---|---|
| With zinc trifluoromethanesulfonate In toluene for 48h; Inert atmosphere; Reflux; | 93% |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
576-83-0
2,4,6-trimethylphenyl bromide

-
-
2571-52-0
cyanomesitylene

| Conditions | Yield |
|---|---|
| Stage #1: 2,4,6-trimethylphenyl bromide With magnesium; lithium chloride In tetrahydrofuran at 23℃; Stage #2: 2,2-dimethylmalononitrile In tetrahydrofuran at 0 - 23℃; for 0.5h; | 93% |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
16932-45-9
1-bromo-2,6-dimethoxybenzene

-
-
16932-49-3
2,6-dimethoxybenzonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 1-bromo-2,6-dimethoxybenzene With magnesium; lithium chloride In tetrahydrofuran at 23℃; Stage #2: 2,2-dimethylmalononitrile In tetrahydrofuran at 0 - 23℃; for 0.5h; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: p-methoxybenzylnitrile With methylmagnesium bromide; lithium chloride In tetrahydrofuran; diethyl ether at 20℃; for 0.5h; Inert atmosphere; Stage #2: 2,2-dimethylmalononitrile In tetrahydrofuran; diethyl ether at 80℃; for 6h; Inert atmosphere; Stage #3: 1-iodo-butane In tetrahydrofuran; diethyl ether; N,N-dimethyl-formamide at 80℃; for 16h; Inert atmosphere; | 93% |

-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
586-77-6
4-bromo-N,N-dimethylaniline

-
-
1197-19-9
4-cyano-N,N-dimethylaniline

| Conditions | Yield |
|---|---|
| Stage #1: 4-bromo-N,N-dimethylaniline With magnesium; lithium chloride In tetrahydrofuran at 23℃; Stage #2: 2,2-dimethylmalononitrile In tetrahydrofuran at 0 - 23℃; for 1h; | 92% |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
99725-44-7
3,5-dimethyl-4-fluoro-1-bromobenzene

-
-
867367-02-0
4-fluoro-3,5-dimethylbenzonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 3,5-dimethyl-4-fluoro-1-bromobenzene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.0833333h; Stage #2: 2,2-dimethylmalononitrile In tetrahydrofuran; hexane at -78 - 20℃; for 1h; | 92% |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
1679-18-1
4-Chlorophenylboronic acid

-
-
118362-76-8
3-(4-chlorophenyl)-2,2-dimethyl-3-oxopropanenitrile

| Conditions | Yield |
|---|---|
| With 4,4'-dimethyl-2,2'-bipyridines; palladium(II) acetylacetonate; toluene-4-sulfonic acid In water; toluene at 80℃; for 24h; Schlenk technique; | 92% |
| Stage #1: 2,2-dimethylmalononitrile; 4-Chlorophenylboronic acid With [Rh(OH)(cod)]2; N,N,N',N'-tetramethyl-1,8-diaminonaphthalene In 1,4-dioxane at 80℃; for 6h; Inert atmosphere; Stage #2: With hydrogenchloride; water In 1,4-dioxane; tert-butyl methyl ether Inert atmosphere; | 91% |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
101251-09-6
(4-acetylaminophenyl)boronic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2,2-dimethylmalononitrile; (4-acetylaminophenyl)boronic acid With [Rh(OH)(cod)]2; N,N,N',N'-tetramethyl-1,8-diaminonaphthalene In 1,4-dioxane at 80℃; for 6h; Inert atmosphere; Stage #2: With hydrogenchloride; water In 1,4-dioxane; tert-butyl methyl ether Inert atmosphere; | 92% |
-
-
932-31-0
ortho-tolylmagnesium bromide

-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
529-19-1
2-Methylbenzonitrile

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 23℃; for 0.5h; | 91% |
-
-
104-92-7
1-bromo-4-methoxy-benzene

-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
874-90-8
4-methoxybenzonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 1-bromo-4-methoxy-benzene With TurboGrignard In tetrahydrofuran; 1,4-dioxane at 0℃; for 48h; Stage #2: 2,2-dimethylmalononitrile In tetrahydrofuran; 1,4-dioxane at 0 - 23℃; for 0.5h; | 91% |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
3975-77-7
1-bromo-2,4,6-tri-tert-butylbenzene

-
-
24309-22-6
2,4,6-tri-(tert-butyl)benzonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 1-bromo-2,4,6-tri-tert-butylbenzene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.25h; Stage #2: 2,2-dimethylmalononitrile In tetrahydrofuran; hexane at -78 - 23℃; for 1h; | 91% |
-
-
7321-55-3
2,2-dimethylmalononitrile

-
-
13922-41-3
1-Naphthylboronic acid

| Conditions | Yield |
|---|---|
| With 4,4'-dimethyl-2,2'-bipyridines; palladium(II) acetylacetonate; toluene-4-sulfonic acid In water; toluene at 80℃; for 24h; Schlenk technique; | 91% |
| With 6,6'-dimethyl-2,2'-bipyridine; p-nitrobenzenesulfonic acid; water; palladium(II) acetylacetonate In toluene at 95℃; for 20h; | 86% |
Propanedinitrile,2,2-dimethyl- Specification
The Propanedinitrile,2,2-dimethyl-, with the CAS registry number 7321-55-3, has the systematic name of dimethylpropanedinitrile. It belongs to the following product categories: C1 to C5; Cyanides/Nitriles; Nitrogen Compounds. And the molecular formula of the chemical is C5H6N2.
The characteristics of Propanedinitrile,2,2-dimethyl- are as followings: (1)ACD/LogP: 0.20; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.2; (4)ACD/LogD (pH 7.4): 0.2; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 30.48; (8)ACD/KOC (pH 7.4): 30.48; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 47.58 Å2; (13)Index of Refraction: 1.424; (14)Molar Refractivity: 25 cm3; (15)Molar Volume: 97.8 cm3; (16)Polarizability: 9.91×10-24cm3; (17)Surface Tension: 37.8 dyne/cm; (18)Density: 0.961 g/cm3; (19)Flash Point: 77.8 °C; (20)Enthalpy of Vaporization: 40.59 kJ/mol; (21)Boiling Point: 169.5 °C at 760 mmHg; (22)Vapour Pressure: 1.54 mmHg at 25°C.
Preparation of Propanedinitrile,2,2-dimethyl-: This chemical can be prepared by malononitrile and iodomethane. The reaction will need reagent potassium tert-butoxide, and the catalyst tetrabutylammonium bromide. The reaction time is 2 hours with ambient temperature, and the yield is about 76%.
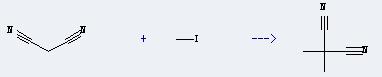
Uses of Propanedinitrile,2,2-dimethyl-: It can react with benzene-1,2-diamine; dihydrochloride to produce 2,2-bis(benzimidazole-2-yl)propane. And the yield is about 70%.
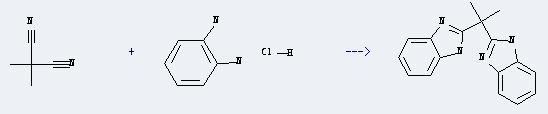
You should be cautious while dealing with this chemical. It is a kind of flammable chemical which irritates to eyes, respiratory system and skin, and it is also toxic by inhalation, in contact with skin and if swallowed. Therefore, you had better take the following instructions: Keep away from sources of ignition - No smoking; Wear suitable protective clothing, gloves and eye/face protection, and if in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice; In case of accident or if you feel unwell, seek medical advice immediately (show label where possible).
Addtionally, the following datas could be converted into the molecular structure:
(1)SMILES: N#CC(C#N)(C)C
(2)InChI: InChI=1/C5H6N2/c1-5(2,3-6)4-7/h1-2H3
(3)InChIKey: BCMJJXWXMZYZKN-UHFFFAOYAH
Related Products
- Propanedinitrile, (4,4-dimethyl-5-((((methyl((trichloromethyl)thio)amino)carbonyl)oxy)imino)-1,3-dithiolan-2-ylidene)-
- Propanedinitrile, 2,2'-(2,5-dimethyl-2,5-cyclohexadiene-1,4-diylidene)bis-
- Propanedinitrile,2-(1,1-dimethylethyl)-
- Propanedinitrile,2-(1,3-dihydro-1,3-dioxo-2H-inden-2-ylidene)-
- Propanedinitrile,2-(1-methylethylidene)-
- Propanedinitrile,2-(2-phenylhydrazinylidene)-
- Propanedinitrile,2-(9H-fluoren-9-ylidene)-
- Propanedinitrile,2-(hydroxyimino)-, sodium salt (1:1)
- Propanedinitrile,2,2-dimethyl-
- Propanedinitrile,2-[(2,3,6,7-tetrahydro-1H,5H-benzo[ij]quinolizin-9-yl)methylene]-
- 73217-31-9
- 73217-75-1
- 73218-79-8
- 7321-93-9
- 73219-89-3
- 73219-91-7
- 73219-92-8
- 73220-38-9
- 73220-53-8
- 73221-18-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View