Synthetic route
-
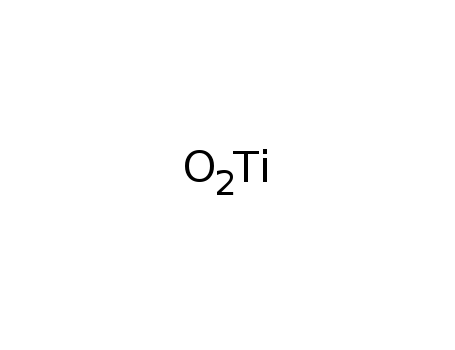
-
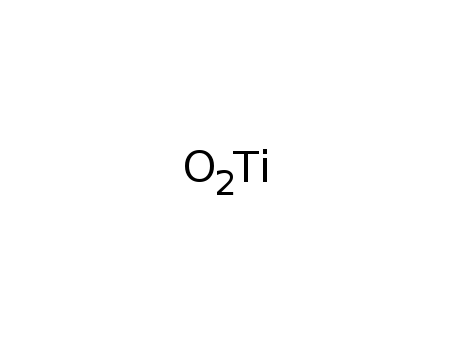
titanium(IV) oxide
-
B

-
calcium oxide
Conditions
| Conditions | Yield |
|---|
| With calcium chloride; calcium oxide In neat (no solvent) mixt. with flux and binder using stirrer; flux was CaCl2 or CaO powder; binder collodion soln. consisted of 5 mass% nitrocellulose in ethanol and ether; | A 99%
B n/a |
-

-
titanium(IV) oxide
Conditions
| Conditions | Yield |
|---|
| With 29.4 percent Na-70.6 percent Ca alloy 83 % pure Ti; | 97% |
| With 29.4 percent Na-70.6 percent Ca alloy 83 % pure Ti; | 97% |
| With calcium 82.5 % pure Ti; | 95.8% |
Conditions
| Conditions | Yield |
|---|
| With Mg byproducts: MgCl2; under Ar, 700-1060°C; washed with water, aq.HCl, alcohol, dried at 120°C, molten in electric arc oven; | 95.9% |
| With methane In gas byproducts: C; Ar; flow-type quartz reactor at 973 K; an aliquot of the chloride was placed in a vaporizer that was at the boiling point of the chloride; together with the methane stream, the chloride was fed from the vaporier to the reactor;; | |
| Electrolysis; in salt melt; | |
-

-
titanium(IV) oxide
Conditions
| Conditions | Yield |
|---|
| In melt Electrolysis; Ti dust pellets sintered at 900°C for 2 h; pellet wrapped with Momesh and wires; heated in air at 330°C for 24 h; electrolysis at 3.1 V in molten CaCl2 at 900°C for 12 h (Ar); cathode lifted ffrom molten salt, cooled in Ar stream, washed in distd. water and dreid in air; detn. by SEM, EDX, XRD, oxygen anal.; | |
-

-
titanium(IV) oxide
-
B
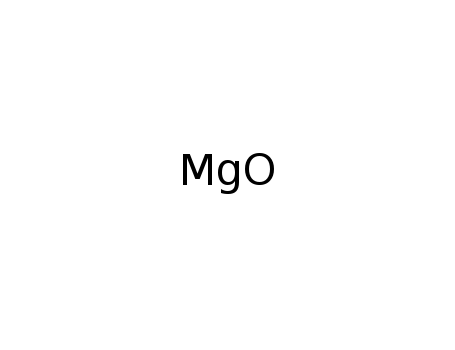
-
magnesium oxide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) High Pressure; TiO2 and Mg were heated at 750°C for 5 h under autogenic pressure; react. mixt was gradually cooled to room. temp. for 5 h, MgO was removedby aq. AcOH for 1 day, ppt. was centrifugated, washed with water and Et OH, and dried, washe with formic acid for 2 days, washed and dried; | |
| With NaCl In neat (no solvent) High Pressure; mixt. of TiO2, magnesium powder, and NaCl pellitized; metallic Ti prepd.by method of self-propogation high-temp. stynthesis in reactor under Ar; detn. by XRD; | |
-
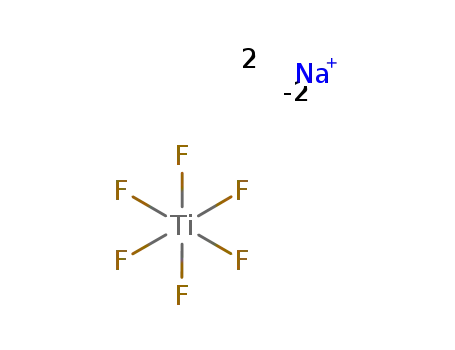
-
sodium hexafluorotitanate
Conditions
| Conditions | Yield |
|---|
| With sodium purification via TiI4; | |
| With Na purification via TiI4; | |
-
titanium hydride
-
titanium hydride
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) byproducts: H2; decomposition at 850°C; | |
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent, solid phase) byproducts: Al, H2, LiCl; mixing of TiCl3 and aluminium compd., ball milling for 20 min in H2; Easton D.S., Schneibel J.H., Speakman S.A. J. Alloys Compd. 2005, 398, 245-248; Balema V., Wiench J.W., Dennis K., Pruski M., Pecharsky V.J. J. Alloys Compd. 2001, 329, 108-114; | |
-

-
titanium carbide
-
B
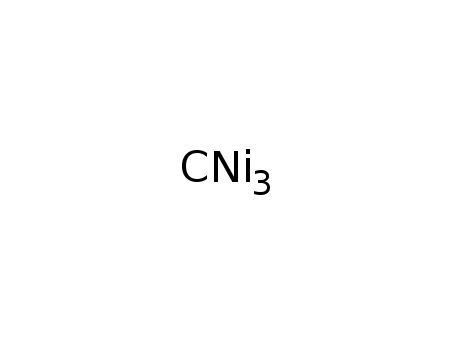
-
nickel carbide
Conditions
| Conditions | Yield |
|---|
| equilibrium; | |
| equilibrium; | |
-

-
titanium carbide
-

-
titanium(IV) oxide
-
B
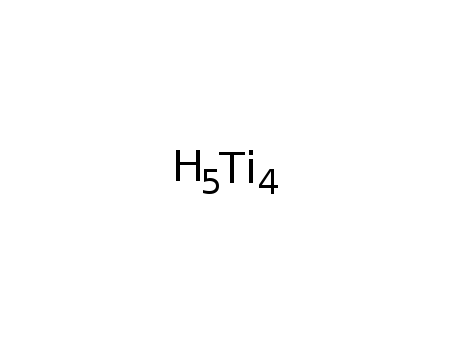
-
Ti4H5
Conditions
| Conditions | Yield |
|---|
| With H2 H2-stream, 400 °C; leading over TiCl4-vapour below 350 ° C, treating with alcohol and HCl; | |
-

-
titanium molybdenum wire
Conditions
| Conditions | Yield |
|---|
| vapourized on heating to 1700°C; | |
-
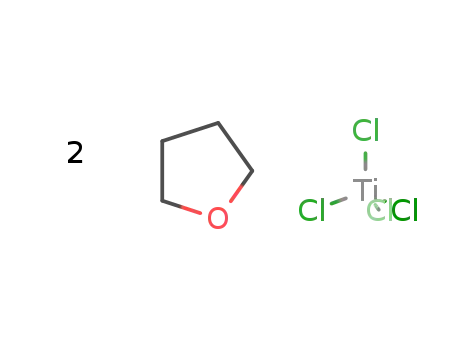
-
titanium(IV) chloride tetrahydrofuran
Conditions
| Conditions | Yield |
|---|
| With K[BEt3H] In tetrahydrofuran byproducts: KCl; under Ar, Schlenk technique; soln. of K(BEt3H) (80 mmol) added dropwise to suspn. of TiCl4*2THF (20 mmol) (room temp., 2 h); stirred (2 h, room temp.); KCl filtered off; solvent removed (vac.); solid dissolved in fresh THF; added to suspn. of SiO2 in THF; stirred (8 h); dried (vac., 24 h); | |
-
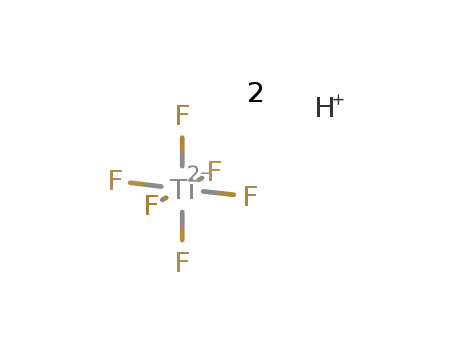
-
hexafluorotitanic acid
Conditions
| Conditions | Yield |
|---|
| Electrolysis; | |
| Electrolysis; | |
-

-
titanium diboride
-
B
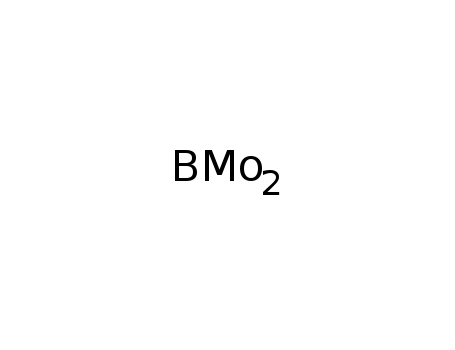
-
molybdenum boride
Conditions
| Conditions | Yield |
|---|
| In 1,2-dimethoxyethane addn. of Mg to suspn. of TiCl3 in DME, refluxing (12 h); | |
-
B

-
titanium chloride
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) Mg film exposing to TiCl4 at 1E-7 and 1E-6 Torr; monitoring by XPS; | |
Conditions
| Conditions | Yield |
|---|
| With Ti oxide In neat (no solvent) thermite process; exclusion of air; mixture of finely dispersed Al and metal oxide is locally ignited by ignition mixture; strong evolution of heat;; mixture of molten Al2O3 and metal obtained;; | |
| With Ti oxide In neat (no solvent) thermite process; exclusion of air; mixture of finely dispersed Al and metal oxide is locally ignited by ignition mixture; strong evolution of heat;; mixture of molten Al2O3 and metal obtained;; | |
-
-
rutile
-
C

-
titanium aluminide
-
D
titanium oxide
-
titanium oxide
-
E

-
Ti2Al
Conditions
| Conditions | Yield |
|---|
| In solid pressing disks of TiO2 and Al together, heating at 646-1200°C; further products: Ti2O3, TiO, TiAl; not isolated, X-ray diffraction; | |
-

-
titanium(IV) oxide
Conditions
| Conditions | Yield |
|---|
| at 950°C in 20 minutes; | |
| at 950°C in 20 minutes; | |
-

-
titanium(IV) oxide
Conditions
| Conditions | Yield |
|---|
| in electric oven; | |
| in electric oven; | |
-

-
rutile
-
-
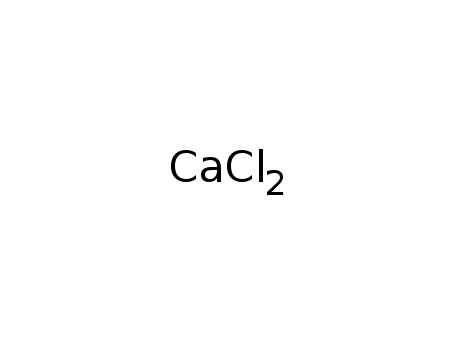
calcium chloride
Conditions
| Conditions | Yield |
|---|
| With CaO In melt Electrochem. Process; metalic titanium prepd. by electrochemical redn. of rutile in CaCl2-CaO melt at temp. 840-960°C under Ar in diaphragm electrolyzer; XRD; | |
-

-
titanium(IV) oxide
-
C

-
titanium(II) hydride
Conditions
| Conditions | Yield |
|---|
| 50% excess of Ca; 99% pure Ti; | |
-

-
titanium(IV) oxide
Conditions
| Conditions | Yield |
|---|
| byproducts: H2; excess CaH2, Ar or He atm.; evacueted to remove H2 and Ca; | |
-

-
titanium(IV) oxide
Conditions
| Conditions | Yield |
|---|
| With hydrogen 600-700°C; washed with 30 % CH3COOH, then 1100°C in vac.; | |
| With H2 600-700°C; washed with 30 % CH3COOH, then 1100°C in vac.; | |
| With H2 | |
-

-
rutile
-
B

-
titanium carbide
Conditions
| Conditions | Yield |
|---|
| With pyrographite Electric Arc; TiO2:C=1:2; 1000-2000 A, 60-70 V; 2% C containing Ti; | |
| With C | |
-

-
rutile
Conditions
| Conditions | Yield |
|---|
| With calcium In further solvent(s) Electrochem. Process; 20 V, 180 A, current density at the cathode 13 A/cm^2 at red heat in CaCl2 bath; prod. containing CaCl2, CaCO3, Fe and TiO2 but free from N and C; | |
| With silicon 3 % Fe, 20.4 % Si; | |
| With Al In neat (no solvent, solid phase) byproducts: Al2O3; ball milled at room temp. under Ar; XRD; | |
| With Ca | |
| With Si | |
-

-
rutile
-
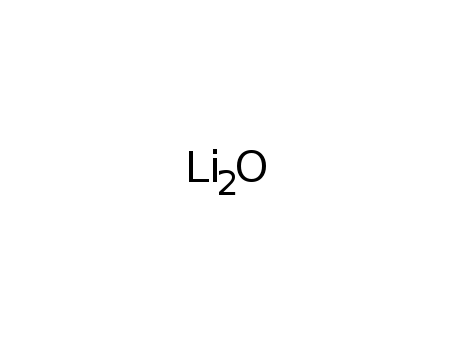
-
lithium oxide
Conditions
| Conditions | Yield |
|---|
| In melt Electrochem. Process; (Ar); TiO2 powder kept in a magnesia holder made the cathode and chargedinto molden LiCl-Li2O at 650 °C at constant current 1.2 A; opera tion voltage lower than decomposition potential of LiCl=-3.46 V but higher than that of Li2O=-2.47 V; | |
-
phosphoric acid
-
phosphoric acid
-
-
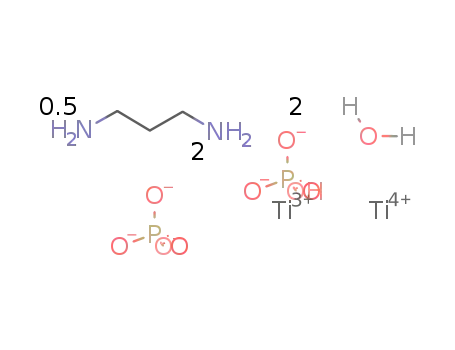
Ti2(PO4)(HPO4)2*(water)2*0.5(1,3-diaminopropane)
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) Ti:1,3-diaminopropane:H3PO4:H2O ratio = 2:2.21:13.65:500, autoclave, pH=2, 170°C (1 d, autogenous pressure); washing (water, alcohol and acetone); elem. anal.; | 100% |
| With boric acid; HF In neat (no solvent) Ti:boric acid:H3PO4:1,3-diaminopropane:HF:H2O ratio = 1:6.54:13.65:2.21:3.36:600; | |
-

-
hafnium
-

-
niobium
-

-
Hf0207Nb0.21Ti0191V0.187Zr0206
Conditions
| Conditions | Yield |
|---|
| Inert atmosphere; Electric arc; | 99.9% |
-

-
titanium nitride
Conditions
| Conditions | Yield |
|---|
| With nitrogen; hydrogen 1000 °C; 10h 1400 °C; | 99.6% |
| With N2; H2 1000 °C; 10h 1400 °C; | 99.6% |
| With nitrogen heating in N2 gas, 800 ° C; | |
-

-
TiCl3*4(CH3)2CHOH
Conditions
| Conditions | Yield |
|---|
| With hydrogen chloride In ethanol argon atmosphere; alcoholic HCl soln. addn. to metal powder, heating (boiling 5-6 h, gentle HCl stream), crystn., crystals transfer into flask with relux condenser, isopropanol addn., mixt. heating (boiling for 4-5 h), crystn. at room temp.; crystals sepn. from mother liquor, residual solvent removal (vac., room temp., 1.5-2 h); elem. anal.; | 99% |
-
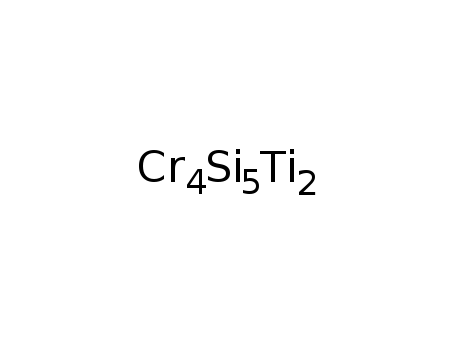
-
Ti2Cr4Si5
Conditions
| Conditions | Yield |
|---|
| In melt Ti, Cr, and Si were pressed into pellets and arc-melted under Ar; X-ray powder diffraction; | 99% |
-

-
pyridinium poly(hydrogenfluoride)
-
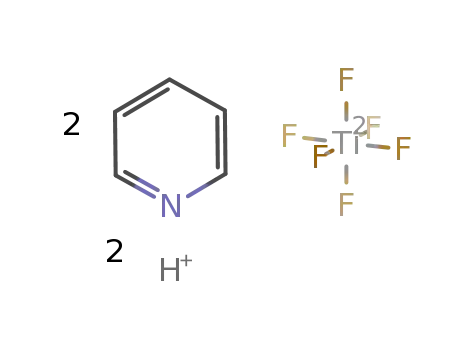
-
pyridinium hexafluorotitanate(IV)
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) byproducts: HF, H2; stirring (4 d); pptn. ((CH3)2O), filtn., washing ((CH3)2O), drying (vac.); | 97% |
-

-
V03429(41)Ti018(24)Mn053
-

-
V02756(45)Ti085(26)Mn059
Conditions
| Conditions | Yield |
|---|
| In melt Electric arc; Inert atmosphere; | A 97%
B n/a |
-
-
graphite
-

-
titanium-aluminium carbide
Conditions
| Conditions | Yield |
|---|
| With Sn In neat (no solvent) other Radiation; mixt. milled for 2 h; coldly pressed into rods; combustion reaction carried out in CO2 laser; XRD; | 95% |
| In solid self-propagating high-temperature synthesis; | |
-
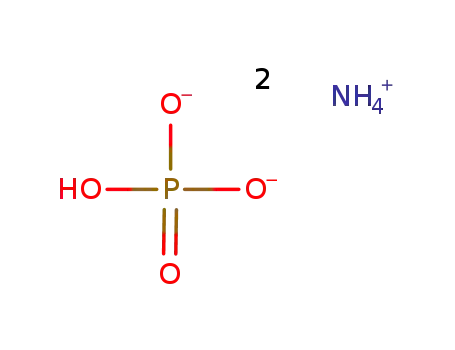
-
diammonium hydrogenphosphate
-

-
titanium(IV) oxide
-
Li2.72Ti2(PO4)2
-
Li2.72Ti2(PO4)2
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent, solid phase) heating mixture of Li2CO3, TiO2 and (NH4)2HPO4 (ratio 1.40:1.15:2.00) at 350°C overnight and at 550°C for 5h, addn. of Ti, heating at 900°C for 3 days (fused silica tube); | 95% |
Conditions
| Conditions | Yield |
|---|
| In melt equimolar mixt. of Ti, As and Te with LiCl-KCl flux sealed in fused silica tube under 1E-4 Torr of Ar; heated at 923 K for 12 h; kept at 923 K for 72 h; cooled to 298 K (3 K/h); washed with water; dried with acetone; detd. by EDX and X-ray diffraction; | 90% |
-

-
cesium iodide
-
A
-
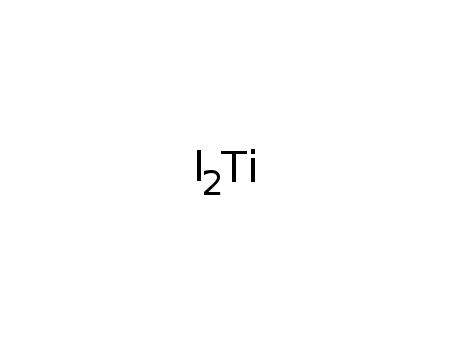
titanium(II) iodide
-
B

-
CsTi5I11
Conditions
| Conditions | Yield |
|---|
| In not given 7 d, 600°C; | A n/a
B 90% |
-
-
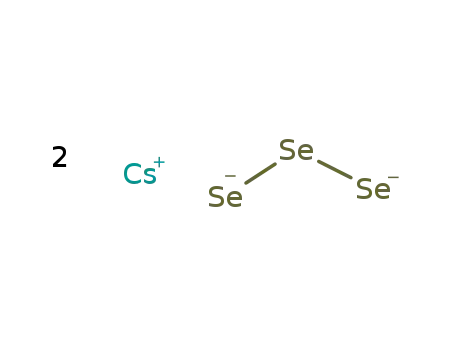
2Cs(1+)*Se3(2-)=Cs2Se3
-

-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper
-

-
Cs2TiCu2Se4
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) Cs2Se3, Ti, Cu, and Se in vacumated tube were heated to 823 K ar 1 K/min, kept at 823 K for 2 days, slowly cooled at 0.10 K/min to 373 K, and then cooled to room temp.; washed with DMSO and dried with acetone; elem. anal.; | 90% |
-

-
dipotassium trisulfide
-

-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) K2S3, Ti, Cu, and S in vacuumated tube were heated to 823 K ar 1 K/min, kept at 823 K for 2 days, slowly cooled at 0.10 K/min to 373 K, and thencooled to room temp.; washed with DMSO and dried with acetone; elem. anal.; | 90% |
-
caesium trisulfide
-
caesium trisulfide
-

-
Cs2TiAg2S4
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) Cs2S3, Ti, Ag, and S in vacumated tube were heated to 823 K ar 1 K/min, kept at 823 K for 2 days, slowly cooled at 0.10 K/min to 373 K, and thencooled to room temp.; washed with DMSO and dried with acetone; elem. anal.; | 90% |
-
A

-
titanium dideuteride
-
B
TiC0.48(2)H0.60
-
TiC0.48(2)H0.60
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) Kinetics; other Radiation; prepd. TiC0.4 pressed, placed into evac. chamber on high-current linear electron accelerator with 4 MeV power and 150 μA; irradn. in vac. at 0.7 Mrad/s for 70 s, then 50 Mrad; after 400 s at 873 K cooling to 373 K, added (2)H2 at 2 bar; at 673 K; detd. by X-ray and neutron diffraction; | A 10%
B 90% |
-
A

-
titanium dideuteride
-
B

-
TiC0.5(2)H0.707
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) Kinetics; other Radiation; mixt. of nominal compn. Ti and C placed into hermetic reactor at const. (2)H2 pressure of 3 bar and ignited locally by heating W spiral; at 200 0 K; detd. by X-ray and neutron diffraction; | A 10%
B 90% |
-
2Rb(1+)*S3(2-)=Rb2S3
-
2Rb(1+)*S3(2-)=Rb2S3
-

-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper
-
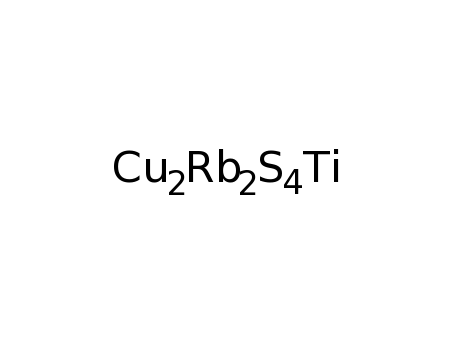
-
Rb2TiCu2S4
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) Rb2S3, Ti, Cu, and S in vacuumated tube were heated to 823 K ar 1 K/min,kept at 823 K for 2 days, slowly cooled at 0.10 K/min to 373 K, and the n cooled to room temp.; washed with DMSO and dried with acetone; elem. anal.; | 90% |
-
-
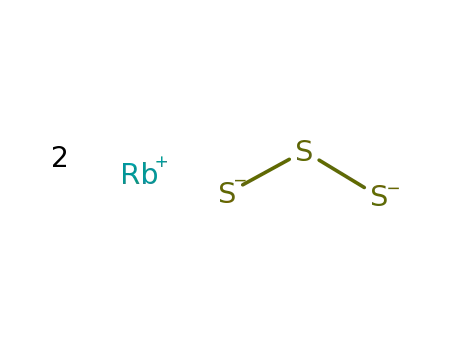
2Rb(1+)*S3(2-)=Rb2S3
-
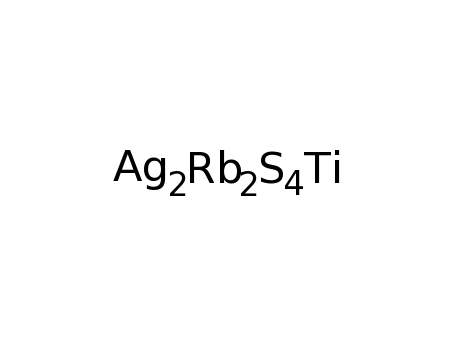
-
Rb2TiAg2S4
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) Rb2S3, Ti, Ag, and S in vacuumated tube were heated to 823 K ar 1 K/min,kept at 823 K for 2 days, slowly cooled at 0.10 K/min to 373 K, and the n cooled to room temp.; washed with DMSO and dried with acetone; elem. anal.; | 90% |
-
-
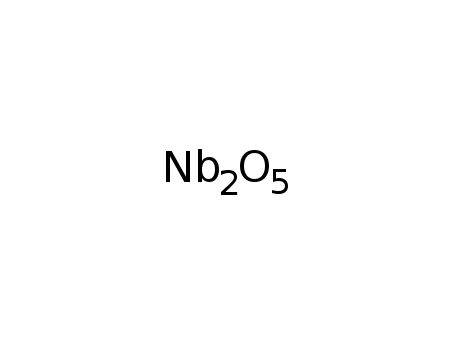
niobium(V) oxide
-

-
niobium
-

-
Ti2Nb6Cl14O4
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) sealed quartz tube (720°C, 60 h), cooling to room temp. within 3 h; | 90% |
-

-
potassium selenide
-

-
phosphorus selenide
-

-
K4Ti2(P2Se9)2(P2Se7)
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) molar ratio Ti:P2Se5:K2Se:Se=1:3:2:10, sealed Pyrex tube (vac.), 490°C, 4 d; cooling to 150°C at 4°C/h; washing (DMF); | 88% |
-

-
rubidium selenide
-

-
phosphorus selenide
-
Rb4Ti2(P2Se9)2(P2Se7)
-
Rb4Ti2(P2Se9)2(P2Se7)
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) molar ratio Ti:P2Se5:Rb2Se:Se=1:3:2:10, sealed Pyrex tube (vac.), 490°C, 4 d; cooling to 150°C at 4°C/h; washing (DMF); | 88% |
-
-
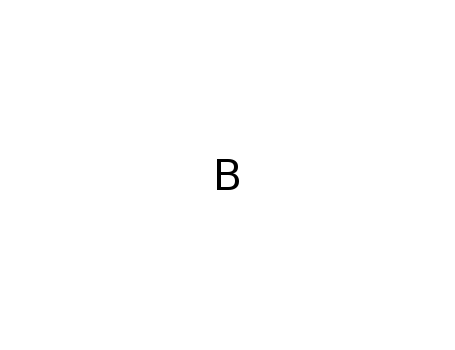
boron
-

-
iridium
-

-
Ti2FeRu2.3Ir2.7B2
Conditions
| Conditions | Yield |
|---|
| In melt Electric Arc; arc melting in water-cooled Cu crucible under Ar using W tip as second electrode; powders pressed into pellet, arc melted for 20 s using direct current of 40 A under Ar; remelted several times; | 87% |
-

-
boron
-

-
iridium
-

-
Ti2FeRu2.8Ir2.2B2
Conditions
| Conditions | Yield |
|---|
| In melt Electric Arc; arc melting in water-cooled Cu crucible under Ar using W tip as second electrode; powders pressed into pellet, arc melted for 20 s using direct current of 40 A under Ar; remelted several times; | 86% |
-
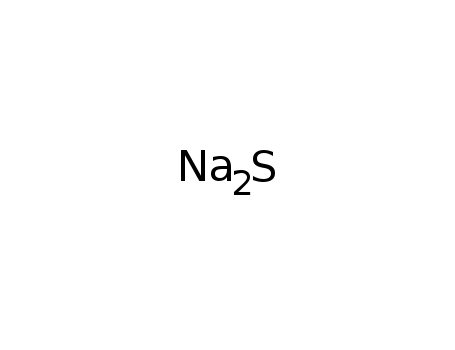
-
sodium sulfide
-
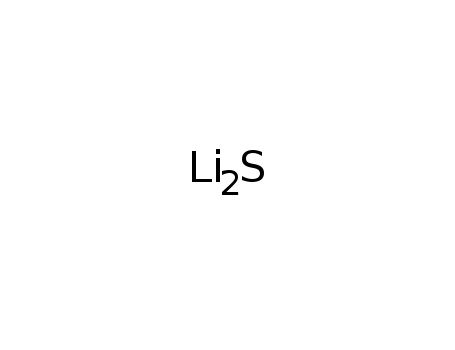
-
lithium sulfide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) mixt. of Ti, S, Li2S, Na2S loaded into silica tube under Ar atm. in glove box; tube sealed under E-4 Torr atm.; placed in furnace; heated to 723K in 24 h; kept at 723 K for 72 h; cooled at 3.5 K/h to 373 K; furnace turned off; washed (DMF); dried (acetone); | 85% |
Titanium Chemical Properties
.jpg)
IUPAC Name: Titanium
Molecular Formula:Ti
Molecular Weight:47.87
EINECS:241-036-9
Solubility: negligible
Classification Code: Reproductive Effect; Tumor data
Stability: Stable. Dust is thought to be spontaneously flammable, and may form an explosive mixture with air. Flammable solid. Incompatible with mineral acids, halogens, carbon dioxide, strong oxidizing agents.
Flash Point: 0 °C
Density (near r.t.): 4.506 g/cm3
Melting point: 1941 K,1668 °C,3034 °F
Boiling point: 3560 K,3287 °C,5949 °F
Heat of fusion: 14.15 kJ/mol
Heat of vaporization: 425 kJ/mol
Specific heat capacity of Titanium (CAS NO.7440-32-6): (25 °C) 25.060 J/mol/K
Titanium Uses
Titanium is used in steel as an alloying element (ferro-titanium) to reduce grain size and as a deoxidizer, and in stainless steel to reduce carbon content. About 95% of titanium ore extracted from the Earth is destined for refinement into titanium dioxide (TiO2), an intensely white permanent pigment used in paints, paper, toothpaste, and plastics. It is also used in cement, in gemstones, as an optical opacifier in paper, and a strengthening agent in graphite composite fishing rods and golf clubs.
Due to their high tensile strength to density ratio, high corrosion resistance, fatigue resistance, high crack resistance, and ability to withstand moderately high temperatures without creeping, titanium alloys are used in aircraft, armor plating, naval ships, spacecraft, and missiles. Due to its high corrosion resistance to sea water, titanium is used to make propeller shafts and rigging and in the heat exchangers of desalination plants; in heater-chillers for salt water aquariums, fishing line and leader, and for divers' knives. Titanium is used to manufacture the housings and other components of ocean-deployed surveillance and monitoring devices for scientific and military use.
Welded titanium pipe and process equipment (heat exchangers, tanks, process vessels, valves) are used in the chemical and petrochemical industries primarily for corrosion resistance. Specific alloys are used in downhole and nickel hydrometallurgy applications due to their high strength titanium Beta C, corrosion resistance, or combination of both. The pulp and paper industry uses titanium in process equipment exposed to corrosive media such as sodium hypochlorite or wet chlorine gas (in the bleachery). Other applications include: ultrasonic welding, wave soldering, and sputtering targets.
Titanium metal is used in automotive applications, particularly in automobile or motorcycle racing, where weight reduction is critical while maintaining high strength and rigidity. The metal is generally too expensive to make it marketable to the general consumer market, other than high-end products, particularly for the racing/performance market. Late model Corvettes have been available with titanium exhausts.
Because it is biocompatible (non-toxic and is not rejected by the body), titanium is used in a gamut of medical applications including surgical implements and implants, such as hip balls and sockets (joint replacement) that can stay in place for up to 20 years.
Titanium Toxicity Data With Reference
| 1. | | ims-rat TDLo:114 mg/kg/77W-I:ETA | | NCIUS* Progress Report for Contract No. PH-43-64-886, Submitted to the National Cancer Institute by the Institute of Chemical Biology, University of San Francisco. (San Francisco, CA 94117) PH 43-64-886,JUL ,1968. |
Titanium Consensus Reports
Reported in EPA TSCA Inventory.
Titanium Safety Profile
Questionable carcinogen with experimental tumorigenic data. Experimental reproductive effects. The dust may ignite spontaneously in air. Flammable when exposed to heat or flame or by chemical reaction. Titanium can burn in an atmosphere of carbon dioxide, nitrogen, or air. Also reacts violently with BrF3, CuO, PbO, (Ni + KClO3), metaloxy salts, halocarbons, halogens, CO2, metal carbonates, Al, water, AgF, O2, nitryl fluoride, HNO3, O2, KClO3, KNO3, KMnO4, steam @ 704°, trichloroethylene, trichlorotrifluoroethane. Ordinary extinguishers are often ineffective against titanium fires. Such fires require special extinguishers designed for metal fires. In airtight enclosures, titanium fires can be controlled by the use of argon or helium. Titanium, in the absence of moisture, burns slowly, but evolves much heat. The application of water to burning titanium can cause an explosion. Finely divided titanium dust and powders, like most metal powders, are potential explosion hazards when exposed to sparks, open flame, or high-heat sources.
Hazard Codes:  F,
F, Xi
Xi
Risk Statements: 20/21/22-11-17-36/38
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.
R11:Highly flammable.
R17:Spontaneously flammable in air.
R36/38:Irritating to eyes and skin.
Safety Statements: 16-36/37/39-33-27-26-6
S16:Keep away from sources of ignition.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S33:Take precautionary measures against static discharges.
S27:Take off immediately all contaminated clothing.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S6:Keep under ... (there follows the name of an inert gas).
RIDADR: UN 2878 4.1/PG 3
WGK Germany: 3
RTECS: XR1700000
F: 10
HazardClass: 4.2
PackingGroup: III
Titanium Analytical Methods
For occupational chemical analysis use NIOSH: Elements (ICP), 7300 or Tissue 8005 (ICP), 8310.
Titanium Specification
Titanium (CAS NO.7440-32-6), its Synonyms are ATI 24 ; C.P. titanium ; CP Titanium ; Contimet 30 ; Oremet ; Smelloff-cutter titanium ; Titanium (dry powder) ; Titanium 50A ; Titanium A-40 ; Titanium VT1 ; Titanium alloy ; Titanium metal powder, dry ; Titanium-125 . Titanium is a gray lustrous powder. It can be easily ignited and burns with an intense flame. The very finely powdered material may be ignited by sparks.
 F,
F, Xi
Xi



































































































.jpg)
 F,
F, Xi
Xi