-
Name
TRIETHYLGALLIUM
- EINECS 214-232-7
- CAS No. 1115-99-7
- Article Data33
- CAS DataBase
- Density 1.058 g/cm3
- Solubility
- Melting Point -82.3 °C
- Formula C6H15Ga
- Boiling Point 143 °C
- Molecular Weight 156.908
- Flash Point -18 °C
- Transport Information UN 3203
- Appearance
- Safety 16-36/37/39-43
- Risk Codes 14/15-17
-
Molecular Structure
- Hazard Symbols F,C
- Synonyms Triethylgallane;
- PSA 0.00000
- LogP 2.54090
Synthetic route

-
-
1115-99-7
triethyl gallium

| Conditions | Yield |
|---|---|
| In neat (no solvent) metal complex decomposed at 120-143°C for 2-3 h; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: triethylaluminum With tri-n-propylamine at 20℃; Stage #2: gallium(III) trichloride at 100℃; for 2h; Neat (no solvent); | 92% |
| In hexane at 70℃; Glovebox; Inert atmosphere; |

-
-
1115-99-7
triethyl gallium

| Conditions | Yield |
|---|---|
| In neat (no solvent) metal complex decomposed at 120-143°C for 2-3 h; | 92% |

-
-
1115-99-7
triethyl gallium

| Conditions | Yield |
|---|---|
| In neat (no solvent) metal complex decomposed at 120-125°C for 2-3 h; | 90% |

-
A
-
1115-99-7
triethyl gallium

-
B
-
30914-08-0
diethylgallium chloride

| Conditions | Yield |
|---|---|
| With potassium chloride; sodium chloride at 120 - 130℃; under 225.023 Torr; Pressure; Concentration; Temperature; Inert atmosphere; | A 75.9% B n/a |
| With potassium chloride; sodium chloride at 120℃; under 225.023 Torr; Inert atmosphere; | A 75.9% B 19% |


-
-
1115-99-7
triethyl gallium

| Conditions | Yield |
|---|---|
| In neat (no solvent) heated slowly under 1E-2 mmHg in a trap-to-trap distillation apparatus (receiver flask at -196 °C), liberation of Et3Ga started at 80 °C, the rate was more satisfactory at 120-125 °C;; | 66% |
-
-
7440-55-3
gallium

-
-
7439-95-4
magnesium

-
-
75-03-6
ethyl iodide

-
A
-
1115-99-7
triethyl gallium

-
B
-
7786-30-3
magnesium chloride

| Conditions | Yield |
|---|---|
| In hexane inert condition; addn. of hexane to mixt. of Mg and Ga (vac.), addn. of hexane, dropwise addn. of EtI (boiling hexane); distillation; | A 65% B n/a |

-
-
7440-55-3
gallium

-
-
7553-56-2
iodine

-
-
7439-95-4
magnesium

-
-
75-03-6
ethyl iodide

-
A
-
1115-99-7
triethyl gallium

-
B
-
7786-30-3
magnesium chloride

| Conditions | Yield |
|---|---|
| In neat (no solvent) inert condition; addn. of I2 to mixt. of Mg and Ga, heating in vac. (150°C), chilling, addn. of EtI (50 - 70°C, 1 h), heating (90 °C, 4 h); distillation; | A 61% B n/a |


| Conditions | Yield |
|---|---|
| In neat (no solvent) inert condition; addn. of I2 to alloy Mg-Ga, heating in vac. (150 °C), chilling, addn. of EtI), heating (85°C, 4 h); distillation; | A 57% B n/a |


| Conditions | Yield |
|---|---|
| With iodine; magnesium In neat (no solvent) byproducts: MgI2; inert atm.; heating (85°C); distn.; | 57% |
-
-
851961-39-2
1,3,5-tris(3,3-dimethyl-1-butynyl)benzene

-
-
93481-56-2
diethylgallium hydride

-
B
-
1115-99-7
triethyl gallium

| Conditions | Yield |
|---|---|
| In hexane under Ar; soln. of Et2GaH in n-hexane added to soln. of C6H3(C2CMe3)3 inn-hexane at room temp.; heated under reflux for 16 h; filtered; filtrate concd. under vac. at room temp.; ppt. collected; | A 44% B n/a |


| Conditions | Yield |
|---|---|
| With gallium(III) trichloride; sodium chloride |

| Conditions | Yield |
|---|---|
| With gallium at 165℃; |

| Conditions | Yield |
|---|---|
| at 165℃; |


| Conditions | Yield |
|---|---|
| With gallium(III) trichloride at 50 - 100℃; for 1 - 3h; Heating / reflux; |

| Conditions | Yield |
|---|---|
| With iodine; magnesium In hexane byproducts: MgI2; inert atm.; boiling; distn.; | |
| With magnesium In neat (no solvent) byproducts: MgI2; inert atm.; heating (90°C, 4 h); distn.; |
-
-
230310-74-4
Et2Ga(NH[C6H2(2,4,6-t-Bu)3])

-
B
-
1115-99-7
triethyl gallium

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: H2N[C6H2(t-Bu)3]; Ar-atmosphere; heating at 65°C for 12 h; distillation off of GaEt3 (vac., room temp.), sublimation off of amine, recrystn. (pentane); elem. anal.; |

| Conditions | Yield |
|---|---|
| With Mg In diethyl ether (N2), EtBr added dropwise to mixt. of Mg and abs.Et2O, boiled under stirring for 2 h, treated dropwise with GaCl3 in Et2O, boiled under stirringfor 1 h; evapd. at 45°C, condensed into liquid N2, separated (refluxer), recruficated; |

-
A
-
188685-27-0
Sn(C6H3-2,6-(C6H2-2,4,6-(CH3)3)2)2

-
B
-
1115-99-7
triethyl gallium

| Conditions | Yield |
|---|---|
| In toluene at 20℃; |

| Conditions | Yield |
|---|---|
| In diethyl ether |

| Conditions | Yield |
|---|---|
| Stage #1: ethyl bromide With magnesium Stage #2: gallium(III) fluoride In diethyl ether for 48h; Reflux; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) all manipulations under N2 atm.; mixed equimolar amts. of Ga and Bi compds. at ambient temp.; elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) under N2 atm. react. Et3Ga and Et4Sb2; elem. anal.; | 100% |

-
-
1115-99-7
triethyl gallium

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 24h; Temperature; Schlenk technique; Inert atmosphere; | 100% |

-
-
1115-99-7
triethyl gallium

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 24h; | 100% |

| Conditions | Yield |
|---|---|
| With hydrogen In toluene High Pressure; a solns. mixed under inert atm., stirred at 130°C for 16 h under H2 pressure (5 MPa), solvent removed, ppt. dried (high vac.), hydrogenated for 24 h at 390°C; elem. anal.; | 99.7% |


| Conditions | Yield |
|---|---|
| In pentane Ar-atmosphere; GaEt3:GaCl3 2:1 molar ratio; evapn. (0°C), distillation (60°C, ; | 99% |
| 2/1 comproportionation of Et3Ga and CaCl3; |

| Conditions | Yield |
|---|---|
| In toluene inert atmosphere; GaEt3 addn. to susp. of Al-ethoxide (3:2 molar ratio);reflux (4 h); cooling; filtration; filtrate evapn. (vac.); elem. anal.; | 98% |

-
-
1115-99-7
triethyl gallium

-
-
338963-61-4
(2-pyridylmethyl)(tert-butyldimethylsilyl)amine

| Conditions | Yield |
|---|---|
| In toluene All manipulations under Ar atm.; soln. of org. compd. added dropwise at 0°C to soln. of GaEt3, stirred at room temp. for 12 h; solvent removed. at room temp. in vac.; elem. anal.; | 98% |

-
-
1115-99-7
triethyl gallium

-
-
869318-27-4
[(calix[4]arene monomethyl ether)(triethyl(nitrido)gallium(III))molybdenum(VI)]

| Conditions | Yield |
|---|---|
| In toluene N2, a soln. of Ga compd. (0.52 equiv.) slowly added to a soln. of Mo compd. (0.52 equiv.) at room temp., stirred for 1 h; solvent removed (vac.); elem. anal.; | 98% |
-
-
5966-51-8
1,3-bis-(dimethylamino)propan-2-ol

-
-
1115-99-7
triethyl gallium

-
-
811786-06-8
[(C2H5)2Ga(OCH(CH2N(CH3)2)2)]2

| Conditions | Yield |
|---|---|
| In dichloromethane (N2); using Schlenk techniques; addn. dropwise of HOCH(CH2NMe2)2 (1 equiv.) to soln. of Et3Ga (1 equiv.) in CH2Cl2 at -78°C with stirringfor 0.5 h; slow warming to room temp., stirring for 5 h; removal of solvent in vac., redissolving in toluene and cooling to -20°C; crystn. for 2 days; elem. anal.; | 98% |
| In hexane (N2); using Schlenk techniques; treatment of HOCH(CH2NMe2)2 (1 equiv.) with Et3Ga (1 equiv.) in hexane at room temp.; |

| Conditions | Yield |
|---|---|
| In pentane Ar-atmosphere; GaEt3:GaCl3 1:2 molar ratio; sublimation (room temp.); elem. anal.; | 97.3% |
-
-
3587-64-2
1-Methoxy-2-methylpropan-2-ol

-
-
1115-99-7
triethyl gallium

-
-
811786-09-1
((C2H5)2Ga(OC(CH3)2CH2OCH3))2

| Conditions | Yield |
|---|---|
| In toluene byproducts: ethane; under N2; ligand added dropwise to soln. of Et3Ga (molar ratio 1:1) in toluene at -78°C with stirring over 0.5 h; warmed slowly to room temp.; stirred for 24 h; solvent removed in vac.; redissolved in toluene; cooled to -20°C;crystd. for several d; elem. anal.; | 96% |

| Conditions | Yield |
|---|---|
| In benzene Ar; heated at 60°C; solvent removed (vac., 1 Torr, 50°C); elem. anal.; | 95% |
| In neat (no solvent) Ar; heated at 50°C; elem. anal.; |
-
-
163122-31-4
N,N'-bis(o-hydroxybenzyl)-1,2-diamino-(4,5-dimethyl)benzene

-
-
1115-99-7
triethyl gallium

-
-
166542-84-3
((OC6H4CH2N)2C6H2(CH3)2)Ga(C2H5)(Ga(C2H5)2)2

| Conditions | Yield |
|---|---|
| In toluene byproducts: CH4; stirring (25°C, 15 min), refluxing (8 h); evapn. (vac.); elem. anal.; | 95% |
-
-
1115-99-7
triethyl gallium

-
-
207676-24-2
Ga2(C2H5)4((CH2NCHC6H2(C(CH3)3)2O)2)

| Conditions | Yield |
|---|---|
| In toluene stirring (room temp., 24 h); evapn., recrystn. (toluene, -30°C); | 95% |
-
-
108-16-7
1-methyl-2-N,N-dimethylaminoethanol

-
-
1115-99-7
triethyl gallium

-
-
811786-08-0
[(C2H5)2Ga(OCH(CH3)CH2N(CH3)2)]2

| Conditions | Yield |
|---|---|
| In dichloromethane (N2); using Schlenk techniques; addn. dropwise of HOCH(CH3)CH2NMe2 (1 equiv.) to soln. of Et3Ga (1 equiv.) in CH2Cl2 at -78°C with stirring for 0.5 h; slow warming to room temp., stirring for 5 h; removal of solvent in vac., redissolving in toluene and cooling to -20°C; as solid; elem. anal.; | 95% |
| In hexane (N2); using Schlenk techniques; treatment of HOCH(CH3)CH2NMe2 (1 equiv.)with Et3Ga (1 equiv.) in hexane at room temp.; |
-
-
108-01-0
2-(N,N-dimethylamino)ethanol

-
-
1115-99-7
triethyl gallium

-
-
552851-83-9
((C2H5)2Ga(OCH2CH2N(CH3)2))2

| Conditions | Yield |
|---|---|
| In toluene byproducts: ethane; under N2; ligand added dropwise to soln. of Et3Ga (molar ratio 1:1) in toluene at -78°C with stirring over 0.5 h; warmed slowly to room temp.; stirred for 24 h; solvent removed in vac.; crystd. by standing at room temp. for several d; elem. anal.; | 95% |
-
-
1522-22-1
1,1,1,5,5,5-hexafluoroacetylacetone

-
-
1115-99-7
triethyl gallium

-
-
203209-46-5
Et2Ga(1,1,1,5,5,5-hexafluoro-2,4-pentanedionato)

| Conditions | Yield |
|---|---|
| In pentane byproducts: CH4; Ar-atmosphere; stirring (-196°C to room temp., room temp., 12 h); distg. (vac., -20°C), fractional distg.; elem. anal.; | 94.9% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) all manipulations under N2 atm.; mixed equimolar amts. of Ga and Bi compds. at ambient temp.; dissolved in pentane and storred at -30°C., elem. anal.; | 94% |
-
-
1115-99-7
triethyl gallium

-
-
89332-24-1
(Di-iso-propylsilandiyl)diphosphan

| Conditions | Yield |
|---|---|
| In n-heptane byproducts: ethane; (N2), metal complex added to a stirred soln. of ligand in heptane, reacted for 20 min; warmed, crystd. for 3 days at 6°C, elem. anal.; | 94% |
-
-
1115-99-7
triethyl gallium

-
-
338963-61-4
(2-pyridylmethyl)(tert-butyldimethylsilyl)amine

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: C2H6; All manipulations under Ar atm.; mixt. of org. compd. and GaEt3 heated at 120°C for 20 h; volatiles removed in vac.; elem. anal.; | 94% |
-
-
852471-37-5
(2-pyridylmethyl)di(tert-butylsilyl)amine

-
-
1115-99-7
triethyl gallium

| Conditions | Yield |
|---|---|
| In tetrahydrofuran All manipulations under Ar atm.; GaEt3 added dropwise at 0°C to soln. of org. compd., stirred at room temp. for 12 h; solvent removed at room temp. in vac.; elem. anal.; | 94% |

| Conditions | Yield |
|---|---|
| In toluene at 20℃; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| In toluene at 20℃; Schlenk technique; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| In pentane (Ar); standard vac. line technique; soln. of GaEt3 and Ga(C5H4Me)3 in pentane stirred overnight at room temp.; pentane was removed by vac. distn.; residue was vac. distd.; elem. anal.; | 93.1% |
-
-
18653-98-0
N,N'-bis(2-hydroxybenzyl)ethylenediamine

-
-
1115-99-7
triethyl gallium

| Conditions | Yield |
|---|---|
| In toluene byproducts: CH4; stirring (25°C, 15 min), refluxing (8 h); evapn. (vac.); elem. anal.; | 93% |
-
-
2287-28-7
N,N'-bis(2-hydroxybenzyl)-1,3-diaminopropane

-
-
1115-99-7
triethyl gallium

-
-
166542-82-1
((OC6H4CH2NCH2)2CH2)Ga(C2H5)(Ga(C2H5)2)2

| Conditions | Yield |
|---|---|
| In toluene byproducts: CH4; stirring (25°C, 15 min), refluxing (8 h); evapn. (vac.); elem. anal.; | 93% |
-
-
1115-99-7
triethyl gallium

-
-
338963-61-4
(2-pyridylmethyl)(tert-butyldimethylsilyl)amine

| Conditions | Yield |
|---|---|
| In toluene byproducts: C2H6; All manipulations under Ar atm.; mixt. of org. compd. and GaEt3 in toluene refluxed for 20 h; volatiles removed at room temp. in vac.; elem. anal.; | 93% |

| Conditions | Yield |
|---|---|
| In dichloromethane (N2); using Schlenk techniques; addn. dropwise of HOCH2CH2OMe (1 equiv.)to soln. of Et3Ga (1 equiv.) in CH2Cl2 at -78°C with stirring fo r 0.5 h; slow warming to room temp., stirring for 5 h; removal of solvent in vac., redissolving in toluene and cooling to -20°C; as oil; | 93% |
| In hexane (N2); using Schlenk techniques; treatment of HOCH2CH2OMe (1 equiv.) withEt3Ga (1 equiv.) in hexane at room temp.; as oil; |
Triethylgallium Specification
The IUPAC name of this chemical is Triethylgallium. With the CAS registry number 1115-99-7 and EINECS registry number 214-232-7, it is also named as Gallium, triethyl-. In addition, the molecular formula is C6H15Ga and the molecular weight is 156.91.
Physical properties about this chemical are: (1)Rotatable Bond Count: 3; (2)Exact Mass: 156.042956; (3)MonoIsotopic Mass: 156.042956; (4)Heavy Atom Count: 7; (5)Complexity: 25.7; (6)Covalently-Bonded Unit Count: 1; (7)#Freely Rotating Bonds: 3 .
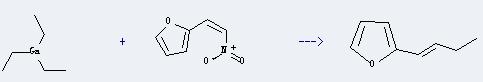
Uses of Triethylgallium: it is used as chemical reagent, fine chemicals, organic synthesis reagents and pharmaceutical intermediates.And it can react with 2-(2-nitro-vinyl)-furan to get 2-but-1-enyl-furan. This reaction will need reagent hexane. The reaction time is 3 hours with ambient temperature. The yield is about 49%.
When you are using this chemical, please be cautious about it as the following:
This chemical is spontaneously flammable in air. And it reacts violently with water, liberating extremely flammable gases. When you are using it, wear suitable protective clothing, gloves and eye/face protection. You should keep away from sources of ignition - No smoking. In case of fire, use ... (indicate in the space the precise type of fire-fighting equipment. If water increases the risk add - Never use water).
You can still convert the following datas into molecular structure:
(1)SMILES: [Ga](CC)(CC)CC
(2)InChI: InChI=1/3C2H5.Ga/c3*1-2;/h3*1H2,2H3;/rC6H15Ga/c1-4-7(5-2)6-3/h4-6H2,1-3H3
(3)InChIKey: RGGPNXQUMRMPRA-KMXMPSPZAO
Related Products
- Triethylgallium
- 111-60-4
- 111613-37-7
- 111613-38-8
- 1116135-66-0
- 1116136-70-9
- 111-61-5
- 1116-22-9
- 111-62-6
- 111628-39-8
- 111631-72-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View