-
Name
Triphosgene
- EINECS 250-986-3
- CAS No. 32315-10-9
- Article Data15
- CAS DataBase
- Density 1.898 g/cm3
- Solubility practically insoluble in water
- Melting Point 78-82 °C
- Formula C3Cl6O3
- Boiling Point 204.5 °C at 760 mmHg
- Molecular Weight 296.749
- Flash Point 53.3 °C
- Transport Information UN 2928 6.1/PG 2
- Appearance White solid
- Safety 36/37/39-45-9-26-36-7/9
- Risk Codes 26-34-29-36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 T+,
T+,  Xn
Xn
- Synonyms Carbonicacid, bis(trichloromethyl) ester (6CI,8CI);Methanol, trichloro-, carbonate(2:1) (9CI);Bis(trichloromethyl) carbonate;Bis(trichloromethyl)carbonate;
- PSA 35.53000
- LogP 3.79500
Synthetic route
-
-
616-38-6
carbonic acid dimethyl ester

-
-
32315-10-9
bis(trichloromethyl) carbonate

| Conditions | Yield |
|---|---|
| With chlorine In tetrachloromethane for 18h; Irradiation; | 100% |
| With chlorine In tetrachloromethane for 28h; Irradiation; | 97% |
| With chlorine for 33h; chlorination; | 88% |
-
-
207804-71-5
carbonic acid chloromethyl ester-dichloromethyl ester

-
-
32315-10-9
bis(trichloromethyl) carbonate

| Conditions | Yield |
|---|---|
| bei der Chlorierung; |
-
-
101970-86-9
methyl 1,1,1-trichloromethyl carbonate

-
C
-
32315-10-9
bis(trichloromethyl) carbonate

| Conditions | Yield |
|---|---|
| Chlorierung; |

-
-
101970-86-9
methyl 1,1,1-trichloromethyl carbonate

-
B
-
32315-10-9
bis(trichloromethyl) carbonate

| Conditions | Yield |
|---|---|
| Chlorierung; |
-
-
101970-86-9
methyl 1,1,1-trichloromethyl carbonate

-
-
32315-10-9
bis(trichloromethyl) carbonate

| Conditions | Yield |
|---|---|
| bei Chlorierung; | |
| durch Chlorierung; |
-
-
79-22-1
methyl chloroformate

-
-
616-38-6
carbonic acid dimethyl ester

-
-
32315-10-9
bis(trichloromethyl) carbonate

| Conditions | Yield |
|---|---|
| vollstaendiges Chlorierung; |
-
-
594-42-3
chlorothio-trichloro-methane

-
-
32315-10-9
bis(trichloromethyl) carbonate

| Conditions | Yield |
|---|---|
| With sulfur dioxide In dichloromethane; water at 10℃; for 4h; |
-
-
1792-81-0
cis-1,2-cyclohexane

-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
4389-22-4, 19456-20-3, 20192-66-9
(3aR,7aS)-hexahydrobenzo[d][1,3]dioxol-2-one

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; | 100% |
| With pyridine In dichloromethane at -70℃; | 90% |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
2216-51-5
(-)-menthol

-
-
14602-86-9
(1R,2S,5R)-menthyl chloroformate

| Conditions | Yield |
|---|---|
| With pyridine In tetrachloromethane at 55 - 60℃; for 6h; | 100% |
| Stage #1: bis(trichloromethyl) carbonate With pyridine In toluene at 0℃; for 0.25h; Inert atmosphere; Stage #2: (-)-menthol In toluene at 20℃; for 15h; Inert atmosphere; | 100% |
| Stage #1: bis(trichloromethyl) carbonate With pyridine In toluene at 0℃; Inert atmosphere; Stage #2: (-)-menthol In toluene at 0 - 20℃; Inert atmosphere; | 100% |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
700-57-2
1-adamantanol

-
-
53120-53-9
2-adamantyl chloroformate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane for 2h; Ambient temperature; | 100% |
| With pyridine In dichloromethane for 1h; Ambient temperature; | |
| With pyridine In dichloromethane; ethyl acetate |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 100% |
| In ethyl acetate for 4h; Heating; | 56% |
| With triethylamine In dichloromethane for 1h; Heating; |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
109-78-4
3-Hydroxypropionitrile

-
-
30436-27-2
β-cyanoethyl chlorocarbonate

| Conditions | Yield |
|---|---|
| Stage #1: bis(trichloromethyl) carbonate; 3-Hydroxypropionitrile In tetrahydrofuran at 0 - 20℃; Stage #2: With pyridine In tetrahydrofuran at 0 - 20℃; | 100% |
| In pyridine; toluene at 0℃; |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
181783-40-4
(-)-(2R,3S,6S)-2-hydroxymethyl-3-methyl-6-phenyl-4-piperidone

-
-
181783-52-8
(2R,3S,6S)-1-aza-3-methyl-6-phenyl-8-oxa-4,7-dioxobicyclo[4.3.0]nonane

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran for 0.25h; | 100% |
-
-
112-53-8
1-dodecyl alcohol

-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
24460-74-0
n-dodecyl chloroformate

| Conditions | Yield |
|---|---|
| With pyridine In tetrachloromethane at -15 - 20℃; | 100% |
| With pyridine In tetrahydrofuran for 2h; | |
| With pyridine In chloroform for 2h; ice cooling; |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
156874-49-6, 169436-70-8, 950744-70-4, 1078151-72-0
5,17-diamino-25,26,27,28-tetrakis(propyloxy)calix[4]arene

-
-
199923-86-9
5,17-bis(isocyanato)-25,26,27,28-tetrapropoxy[4]arene

| Conditions | Yield |
|---|---|
| In toluene Heating; | 100% |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
70367-35-0
cis-2-hydroxycyclohexanecarbonitrile

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; | 100% |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
202340-92-9
(1S,4aR,5S,6S,9R,10R,11S,12S,12aS)-1,12-(Isopropylidenedioxy)-9,12a,13,13-tetramethyl-4-methylenetetradecahydro-6,10-methanobenzocyclodecene-5,6,9,10,11-pentaol

-
-
222727-00-6
(4S,4aS,5S,6S,7R,8R,11S,12S,12aR)-11,12-(Carbonyldioxy)-4,5-(isopropylidenedioxy)-4a,8,13,13-tetramethyl-1-methylenetetradecahydro-7,11-methanobenzocyclodecene-5,7,8-triol

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at -45℃; | 100% |
| With pyridine In dichloromethane at -45℃; for 1.16667h; Acylation; Cyclization; | 100% |
-
-
100-60-7
N-methylcyclohexylamine

-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
35028-38-7
N-methyl-N-cyclohexylaminocarbonyl chloride

| Conditions | Yield |
|---|---|
| With pyridine In toluene | 100% |
| With pyridine In toluene | |
| With pyridine In dichloromethane at 20℃; for 2h; |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
2104-89-4, 2788-84-3, 24184-43-8
D-serine methyl ester

-
-
144542-43-8
(R)-2-oxo-oxazolidine-4-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane 0-5 deg C, 2 h; room temperature, 48 h; | 100% |
| In tetrahydrofuran at 65℃; for 4h; | |
| In tetrahydrofuran at 65℃; for 4h; Condensation; | |
| With potassium carbonate In toluene at 20℃; |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
220595-40-4
labda-12,14-dien-7α,8α-cyclocarbonate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at -78 - 0℃; for 4h; | 100% |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
234117-19-2
(1S,3S)-1-{(1S,2S)-1,3-Bis-(3,4-dimethoxy-phenyl)-2-[(S)-2-(1H-indol-3-yl)-1-methoxycarbonyl-ethylamino]-propyl}-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In dichloromethane at 20℃; Cyclization; | 100% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 100% |
| In ethyl acetate at 0 - 5℃; for 3h; Solvent; Cooling with ice; Reflux; | 89% |
| In chloroform at 20℃; for 1h; Cooling with ice; | 85% |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
540-37-4
p-aminoiodobenzene

-
-
15845-62-2
1-iodo-4-isocyanatobenzene

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 100% |
| In ethyl acetate for 4h; Heating; | 74% |
| With triethylamine In dichloromethane at -35 - 20℃; for 2h; | 60% |
-
-
372-19-0
meta-fluoroaniline

-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
404-71-7
1-fluoro-3-isocyanatobenzene

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 100% |
| With triethylamine In benzene for 3h; Heating; | 75% |
| With triethylamine In benzene acylation; Heating; | 41% |
-
-
348-54-9
2-Fluoroaniline

-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
16744-98-2
1-Fluoro-2-isocyanato-benzene

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 100% |
| With triethylamine In benzene for 3h; acylation; Heating; | 36% |
| With triethylamine In benzene for 3h; Condensation; Heating; | 32% |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
371-40-4
4-fluoroaniline

-
-
1195-45-5
para-fluorophenyl isocyanate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 100% |
| With triethylamine In toluene for 7h; Reflux; Cooling with ice; | 76.6% |
| In 1,4-dioxane at 80℃; for 24h; | 73.1% |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
18144-47-3
tert-butyl 4-aminobenzoate

-
-
100-01-6
4-nitro-aniline

-
-
301317-92-0
1-(4'-nitrophenyl)-3-(4''-tert-butylcarboxyphenyl)urea

| Conditions | Yield |
|---|---|
| Stage #1: bis(trichloromethyl) carbonate; 4-nitro-aniline With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 0 - 20℃; for 0.833333h; Substitution; Stage #2: tert-butyl 4-aminobenzoate In tetrahydrofuran at 20℃; for 42h; Substitution; | 100% |

-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
57260-71-6
1-t-Butoxycarbonylpiperazine

-
-
59878-28-3
tert-butyl 4-(chlorocarbonyl)piperazine-1-carboxylate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; for 2h; Inert atmosphere; | 100% |
| With pyridine In dichloromethane at 0 - 25℃; for 1h; | 89% |
| Stage #1: 1-t-Butoxycarbonylpiperazine With pyridine In dichloromethane at 0℃; for 0.166667h; Inert atmosphere; Stage #2: bis(trichloromethyl) carbonate In dichloromethane at 0℃; for 1h; Inert atmosphere; | 86% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 45℃; for 12h; | 100% |
| In 1,2-dichloro-ethane for 3.25h; Reflux; | 96% |
| In tetrahydrofuran for 4h; Reflux; | 95% |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
7443-52-9
(+/-)-trans-2-methylcyclohexanol

-
-
355023-73-3
allyl α-syn-oximino-α-(2-pyridyl)acetate

| Conditions | Yield |
|---|---|
| Stage #1: bis(trichloromethyl) carbonate; (+/-)-trans-2-methylcyclohexanol With pyridine In dichloromethane at 50℃; for 1.5h; Stage #2: allyl α-syn-oximino-α-(2-pyridyl)acetate With triethylamine In dichloromethane at 25℃; for 1.5h; Further stages.; | 100% |

-
-
579-66-8
2,6-diethylaniline

-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
20458-99-5
2,6-diethylphenylisocyanate

| Conditions | Yield |
|---|---|
| With triethylamine In 1,2-dichloro-ethane Heating; | 100% |
| With triethylamine In 1,2-dichloro-ethane |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
199923-84-7
5-monoamino-25,26,27,28-tetrakis(propyloxy)-calix[4]arene

-
-
496067-40-4
C41H47NO5

| Conditions | Yield |
|---|---|
| In toluene Heating; | 100% |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
498542-65-7
2-[(1S,5S,7R)-1-(tert-Butyl-diphenyl-silanyloxymethyl)-6-aza-spiro[4.5]dec-7-yl]-ethanol

-
-
498542-66-8
C29H39NO3Si

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 100% |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
536-90-3
m-Anisidine

-
-
18908-07-1
3-methoxyphenyl isocyanate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 100% |
| In dichloromethane at 20℃; for 1.5h; | |
| In toluene at 100℃; Cooling with ice; |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
90-04-0
2-methoxy-phenylamine

-
-
700-87-8
1-Isocyanato-2-methoxy-benzene

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 100% |
| In dichloromethane at 20℃; for 1.5h; | |
| In dichloromethane at 0℃; for 0.5h; | |
| With triethylamine In 1,2-dichloro-ethane at 0 - 85℃; for 8.5h; Inert atmosphere; | |
| With triethylamine In 1,2-dichloro-ethane at 0 - 85℃; for 8.5h; Inert atmosphere; |
-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
104-94-9
4-methoxy-aniline

-
-
5416-93-3
4-Methoxyphenyl isocyanate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 100% |
| Stage #1: bis(trichloromethyl) carbonate; 4-methoxy-aniline In dichloromethane at 20℃; for 0.5h; Stage #2: With triethylamine In dichloromethane at -35 - 20℃; for 2h; | 92% |
| In chloroform at 20℃; for 1h; Cooling with ice; | 85% |
Triphosgene Chemical Properties
Molecular Structure of Triphosgene (CAS NO.32315-10-9):
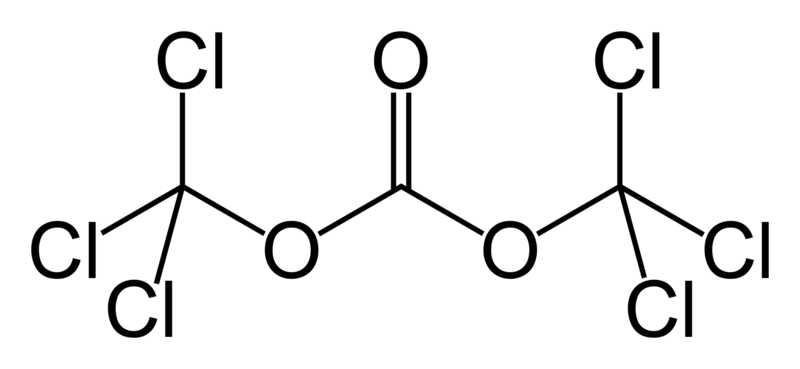
IUPAC Name: bis(trichloromethyl) carbonate
Molecular formula:: C3Cl6O3
Molecular Weight: 296.75 g/mol
H bond acceptors: 3
H bond donors: 0
Freely Rotating Bonds: 2
Polar Surface Area: 35.53Å2
Index of Refraction: 1.532
Molar Refractivity: 48.49 cm3
Molar Volume: 156.3 cm3
Surface Tension: 50.5 dyne/cm
Density: 1.898 g/cm3
Flash Point: 53.3 °C
Enthalpy of Vaporization: 44.07 kJ/mol
Boiling Point: 204.5 °C at 760 mmHg
Vapour Pressure: 0.263 mmHg at 25°C
Melting point: 78-82 °C
Storage temp: 2-8°C
Water Solubility: practically insoluble
Sensitive: Moisture Sensitive
BRN: 1787583
Product Categories: Other Reagents
Triphosgene Production
Triphosgene (CAS NO.32315-10-9) is prepared by exhaustive chlorination of dimethyl carbonate. The preparing process is the chlorination of methyl carbonate in the presence of fluorescence, ultraviolet ray and mixed medium and low temperature initiator in the amount of not more than 2 % of the material. The whole chlorination is completed in three stages at 15-50 deg.c, 50-70 deg.c and 70-90 deg.c separately. The said technological process obtains product of high purity and smelting point of 80-83 deg.c at a yield over 90 %.
Triphosgene Safety Profile
Hazard Codes:  T+,
T+, Xn
Xn
Risk Statements: 26-34-29-36/37/38-20/21/22
R26:Very toxic by inhalation.
R34:Causes burns.
R29:Contact with water liberates toxic gas.
R36/37/38:Irritating to eyes, respiratory system and skin.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.
Safety Statements: 36/37/39-45-9-26-36-7/9
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S9:Keep container in a well-ventilated place.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S7:Keep container tightly closed.
RIDADR: UN 2928 6.1/PG 2
WGK Germany: 2
F: 10-19-21
HazardClass: 6.1
PackingGroup: II
HS Code: 29209010
Triphosgene Specification
Triphosgene , with CAS number of 32315-10-9, can be called Bis(trichloromethyl)carbonate ; Bis(trichloromethyl) carbonate ; Methanol, trichloro-, carbonate(2:1) (9CI) ; Carbonicacid, bis(trichloromethyl) ester (6CI,8CI) . It is a white solid. Triphosgene (CAS NO.32315-10-9) is a chemical compound that is used as a safer substitute for phosgene, because at room temperature it is a solid crystal, as opposed to phosgene which is a gas, the crystals decompose at around 130 °C, although, the decomposition temperature of impure samples can be much lower. It is used in organic synthesis to bond one carbonyl group to two alcohols, as in the synthesis of octalactin B.
Related Products
- Triphosgene
- 32316-92-0
- 323196-43-6
- 323197-73-5
- 32324-19-9
- 32326-14-0
- 3232-62-0
- 32326-25-3
- 32327-48-3
- 32327-52-9
- 32327-71-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View