-
Name
m-Phthalaldehyde
- EINECS 210-935-8
- CAS No. 626-19-7
- Article Data82
- CAS DataBase
- Density 1.189 g/cm3
- Solubility Slightly soluble in water.
- Melting Point 87-88 °C(lit.)
- Formula C8H6O2
- Boiling Point 255.3 °C at 760 mmHg
- Molecular Weight 134.134
- Flash Point 94.1 °C
- Transport Information
- Appearance colourless or light yellow crystals
- Safety 22-24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Isophthalaldehyde(6CI,7CI,8CI);1,3-Benzenedialdehyde;1,3-Diformylbenzene;3-Formylbenzaldehyde;Benzene-1,3-dicarbaldehyde;Isophthaldehyde;Isophthaldialdehyde;NSC 5092;m-Benzenedialdehyde;m-Benzenedicarbaldehyde;m-Benzenedicarboxaldehyde;m-Diformylbenzene;m-Formylbenzaldehyde;
- PSA 34.14000
- LogP 1.31160
Synthetic route

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; molecular sieve In hexane for 3h; Heating; | 98% |
| With manganese(II) nitrate; C70H128N16O4; oxygen; copper(II) nitrate In acetic acid at 20℃; for 4h; Mechanism; | 90% |
| With 2,2,6,6-tetramethyl-piperidine-N-oxyl In ethyl acetate; toluene at 0℃; | 83% |
-
-
167862-23-9
1,3-bis-diacetoxymethyl-benzene

-
-
626-19-7
Isophthalaldehyde

| Conditions | Yield |
|---|---|
| With ethanol at 50 - 60℃; for 0.0833333h; | 95% |
| With rice husk supported FeCl3 nanoparticles In ethanol at 70℃; for 0.583333h; | 90% |
| With N-sulfonic acid poly(4-vinylpyridinium) chloride In methanol at 20℃; for 0.583333h; | 87% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In acetonitrile at 85℃; for 4h; Reagent/catalyst; Temperature; | 94% |
| With C15H11ClN4SZn; p-benzoquinone In tert-butyl alcohol for 8h; Reagent/catalyst; | 72% |
| With tert.-butylhydroperoxide; C29H25Cl2N4Ru(1+)*F6P(1-) In acetonitrile at 60℃; for 3h; Schlenk technique; Inert atmosphere; | 62% |
-
-
626-18-6
1,3-dimethanol benzene

-
A
-
626-19-7
Isophthalaldehyde

-
B
-
52010-98-7
3-(hydroxymethyl)benzaldehyde

| Conditions | Yield |
|---|---|
| With oxygen; potassium carbonate; palladium diacetate In N,N-dimethyl acetamide at 100℃; under 760.051 Torr; for 12h; | A 93% B 3% |
| With cucurbit[8]uril; 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In water for 0.0833333h; Catalytic behavior; Reagent/catalyst; Temperature; Reflux; | A 41% B 43% |
| With manganese(IV) oxide In tetrahydrofuran at 45℃; Rate constant; k2/k1; |

| Conditions | Yield |
|---|---|
| With bis-{4-methoxy-phenyl}-selenoxyde; sodium hydrogencarbonate In acetonitrile at 75℃; for 5h; | 90% |
| Stage #1: 1,3-bis-(bromomethyl)benzene With 2-nitropropane; sodium methylate In methanol; ethyl acetate at 30℃; for 6h; Stage #2: With water In methanol; ethyl acetate | 25.8% |
| With hydrogenchloride; hexamethylenetetramine; acetic acid | |
| Multi-step reaction with 2 steps 2: aqueous NaOH View Scheme |

-
-
626-19-7
Isophthalaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In isopropyl alcohol for 1.5h; Ambient temperature; | 90% |

| Conditions | Yield |
|---|---|
| With pentacoordinated hydrogenosilane 1 | 89% |
| With sodium tris(tert-butoxo)aluminium hydride In tetrahydrofuran; diethylene glycol dimethyl ether at -78℃; for 3h; | 88% |
| With lithium tri-t-butoxyaluminum hydride | |
| Multi-step reaction with 2 steps 1: triethylsilane; triethylamine / tetrahydrofuran / 1 h / 20 °C 2: triethylsilane / tetrahydrofuran / 1 h View Scheme |

-
-
626-19-7
Isophthalaldehyde

| Conditions | Yield |
|---|---|
| With triethylsilane In tetrahydrofuran for 1h; Reagent/catalyst; Fukuyama Reduction; | 89% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-dibromobenzene With tert.-butyl lithium In pentane at -78℃; for 1h; Inert atmosphere; Schlenk technique; Stage #2: N,N-dimethyl-formamide In pentane at -78 - 20℃; Inert atmosphere; Schlenk technique; | 88% |

-
-
626-19-7
Isophthalaldehyde

| Conditions | Yield |
|---|---|
| With sulfonated rice husk ash In acetonitrile at 60℃; for 0.166667h; | 87% |

| Conditions | Yield |
|---|---|
| With nitric acid In m-xylene | 84.9% |

| Conditions | Yield |
|---|---|
| With triethylsilane; palladium diacetate; sodium hydrogencarbonate; sodium carbonate at 20℃; under 760.051 Torr; for 48h; | 84% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-di(aminomethyl)benzene With hexamethylenetetramine In water at 20℃; for 3h; pH=3.6 - 4.5; Stage #2: With hydrogenchloride for 3h; pH=3.5 - 4.5; Reflux; | 83.7% |
| With hydrogenchloride; hexamethylenetetramine; acetic acid |
-
-
54125-02-9
danishefsky's diene

-
-
488081-87-4
3-[(phenylimino)methyl]benzaldehyde

-
A
-
626-19-7
Isophthalaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: danishefsky's diene; 3-[(phenylimino)methyl]benzaldehyde; scandium tris(trifluoromethanesulfonate) In acetonitrile at 0℃; for 0.05h; Diels-Alder reaction; Stage #2: With water In acetonitrile Further stages.; | A 4% B 83% C 3% D 5% |


-
A
-
626-19-7
Isophthalaldehyde

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride In dichloromethane for 0.5h; Ambient temperature; | A 82% B n/a |

-
-
626-19-7
Isophthalaldehyde

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride In dichloromethane at -10 - 20℃; for 0.5h; | 82% |
-
-
36323-28-1
α,α,α',α'-tetrabromo-m-xylene

-
-
626-19-7
Isophthalaldehyde

| Conditions | Yield |
|---|---|
| With dimethyl amine at 60℃; for 2h; | 80% |
| With sulfuric acid at 70 - 110℃; |

-
-
626-19-7
Isophthalaldehyde

| Conditions | Yield |
|---|---|
| With iron(II) fluoride; 1,3-bis(3,3,3-trifluoropropyl)-1,1,3,3-tetramethyldisiloxane In ethanol at 80℃; for 24h; | 80% |

-
-
626-19-7
Isophthalaldehyde

| Conditions | Yield |
|---|---|
| With oxygen In acetonitrile for 24h; Irradiation; | 78% |

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine; 4,4'-dimethoxyphenyl disulfide; iridium(lll) bis[2-(2,4-difluorophenyl)-5-methylpyridine-N,C20]-4,40-di-tert-butyl-2,20-bipyridine hexafluorophosphate; triphenylphosphine In toluene for 24h; Irradiation; | 78% |

| Conditions | Yield |
|---|---|
| With cadmium sulphide In neat (no solvent) at 20℃; for 12h; Irradiation; | 77% |
| With air; Ag/AgBr/TiO2 nanotubes In acetonitrile at 20℃; for 48h; Irradiation; | 69% |

| Conditions | Yield |
|---|---|
| With triethylsilane; palladium diacetate; sodium hydrogencarbonate; sodium carbonate at 20℃; under 760.051 Torr; for 48h; | 76% |

| Conditions | Yield |
|---|---|
| With iodine; triethylamine; triphenylphosphine In toluene at 80℃; for 4h; Sealed tube; | 76% |
| With iodine; triethylamine; triphenylphosphine In toluene at 80℃; for 6h; Sealed tube; Inert atmosphere; | 75% |

| Conditions | Yield |
|---|---|
| With N-hydroxyphthalimide; nitrogen(II) oxide In acetonitrile at 60℃; for 10h; | A 75% B 10% |

| Conditions | Yield |
|---|---|
| With dirhodium tetraacetate; Selectfluor In trifluoroacetic acid; trifluoroacetic anhydride at 80℃; for 7h; Sealed tube; Inert atmosphere; chemoselective reaction; | 71% |

| Conditions | Yield |
|---|---|
| With 1-methyl-piperazine; sodium bis(2-methoxyethoxy)aluminium dihydride In toluene at 5℃; for 1h; | 65.6% |
-
-
52010-98-7
3-(hydroxymethyl)benzaldehyde

-
-
626-19-7
Isophthalaldehyde

| Conditions | Yield |
|---|---|
| With dimethyl sulfoxide UV-irradiation; | 62% |
| With manganese(IV) oxide In tetrahydrofuran at 45℃; Rate constant; |
-
-
4422-95-1
1,3,5-benzene tris(carbonyl chloride)

-
A
-
626-19-7
Isophthalaldehyde

-
B
-
3163-76-6
benzene-1,3,5-trialdehyde

| Conditions | Yield |
|---|---|
| With hydrogen; thiourea; Pd-BaSO4 In xylene for 10h; Heating; | A 19% B 59% |

| Conditions | Yield |
|---|---|
| With palladium(II) acetylacetonate; hydrogen; 2,2-dimethylpropanoic anhydride; dicyclohexylphenylphosphine In tetrahydrofuran at 80℃; under 3750.38 Torr; for 20h; Inert atmosphere; | 42% |
| With hydrogen; 2,2-dimethylpropanoic anhydride; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran at 80℃; under 22501.8 Torr; for 24h; | 94 % Spectr. |
| With hydrogen; 2,2-dimethylpropanoic anhydride; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran at 80℃; under 22501.8 Torr; for 24h; | 94 % Spectr. |
| Multi-step reaction with 2 steps 1: 63 percent / 5-methyl-2-chloro-3-phenyl-2,3-dihydro-1,3,4,2-oxadiazaphosphole, pyridine / Ambient temperature 2: 82 percent / DIBALH / CH2Cl2 / 0.5 h / -10 - 20 °C View Scheme |
-
-
488081-87-4
3-[(phenylimino)methyl]benzaldehyde

-
A
-
626-19-7
Isophthalaldehyde

-
B
-
16742-78-2
N,N'-(1,3-phenylenebis(methan-1-yl-1-ylidene))dianiline

| Conditions | Yield |
|---|---|
| scandium tris(trifluoromethanesulfonate) In [D3]acetonitrile at 0℃; for 1h; | A 23% B 27% |
-
-
23364-44-5
(1S,2R)-2-Amino-1,2-diphenylethanol

-
-
626-19-7
Isophthalaldehyde

-
-
155906-82-4
(1S,2R)-2-{[1-(3-{[(E)-(1R,2S)-2-Hydroxy-1,2-diphenyl-ethylimino]-methyl}-phenyl)-meth-(E)-ylidene]-amino}-1,2-diphenyl-ethanol

| Conditions | Yield |
|---|---|
| With molecular sieve In dichloromethane Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With molecular sieve In dichloromethane Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With molecular sieve In dichloromethane Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With molecular sieve In dichloromethane Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With molecular sieve In dichloromethane Ambient temperature; | 100% |
-
-
626-19-7
Isophthalaldehyde

-
-
161832-74-2
(-)-(S)-2-amino-4-methyl-1,1-diphenylpentanol

-
-
155906-83-5
(S)-2-{[1-(3-{[(E)-(S)-1-(Hydroxy-diphenyl-methyl)-3-methyl-butylimino]-methyl}-phenyl)-meth-(E)-ylidene]-amino}-4-methyl-1,1-diphenyl-pentan-1-ol

| Conditions | Yield |
|---|---|
| With molecular sieve In dichloromethane Ambient temperature; | 100% |
-
-
626-19-7
Isophthalaldehyde

-
-
156457-68-0
thioxobis(1-methylhydrazino)phosphoranyl azide

| Conditions | Yield |
|---|---|
| 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane; trifluoroacetic acid at 20℃; for 48h; | 100% |

| Conditions | Yield |
|---|---|
| at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene at 110℃; for 17h; Dean-Stark; | 100% |

| Conditions | Yield |
|---|---|
| With bromocyane In ethanol at 0 - 20℃; for 0.00138889h; Michael Addition; Sealed tube; stereoselective reaction; | A 100% B n/a |


| Conditions | Yield |
|---|---|
| With 4,4′-(butane-1,4-diyl)bis(1-sulfo-1,4-diazabicyclo[2.2.2]octane-1,4-diium) tetrachloride; ammonium acetate In neat (no solvent) at 100℃; for 0.25h; Hantzsch Dihydropyridine Synthesis; Green chemistry; | 100% |


| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 24h; | 100% |
-
-
626-19-7
Isophthalaldehyde

-
-
141-82-2
malonic acid

-
-
23713-86-2
1,3-phenylene-3,3'-bis(2-propenoic) acid

| Conditions | Yield |
|---|---|
| With piperidine; pyridine at 100℃; | 99% |
| With piperidine; pyridine for 24h; Doebner reaction; Heating; | 91% |
| With pyridine at 50℃; | |
| With sodium acetate; acetic anhydride at 140 - 150℃; | |
| With piperidine; pyridine for 24h; Reflux; |
-
-
626-19-7
Isophthalaldehyde

-
-
189440-33-3
(2-aminoethyl)bis(2-pyridylmethyl)amine

-
-
1227512-79-9
C36H38N8

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 20℃; | 99% |
| In methanol for 24h; Molecular sieve; Inert atmosphere; Reflux; | |
| In tetrahydrofuran at 0 - 20℃; for 15h; |
-
-
626-19-7
Isophthalaldehyde

-
-
149525-64-4
1,3,5-tris(aminomethyl)-2,4,6-trimethylbenzene

-
-
1260940-97-3
C48H48N6

| Conditions | Yield |
|---|---|
| In methanol; dichloromethane at 20℃; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In dichloromethane at 0℃; for 1h; | 99% |

| Conditions | Yield |
|---|---|
| With acetic acid In methanol for 7h; Reflux; | 99% |

| Conditions | Yield |
|---|---|
| With acetic acid In methanol for 7h; Reflux; | 99% |
-
-
617-86-7
triethylsilane

-
-
626-19-7
Isophthalaldehyde

-
-
1376253-63-2
1,3-bis(((triethylsilyl)oxy)methyl)benzene

| Conditions | Yield |
|---|---|
| With C42H43BN3P2(1+)*C18HBF15(1-) In chloroform at 40℃; for 1h; Reagent/catalyst; Temperature; Schlenk technique; Glovebox; | 99% |
| With [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2 In toluene at 50℃; for 15h; Inert atmosphere; | 77% |
| With rhenium(I) pentacarbonyl chloride for 4h; Photolysis; Vacuum; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol at 0 - 20℃; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With acetic acid In N,N-dimethyl-formamide for 0.116667h; Solvent; Microwave irradiation; Reflux; | 99% |

| Conditions | Yield |
|---|---|
| In ethanol | 99% |

| Conditions | Yield |
|---|---|
| In ethanol | 99% |

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 40℃; for 20h; Inert atmosphere; | 99% |
-
-
626-19-7
Isophthalaldehyde

-
-
157379-36-7
N,N'-bis(3-endo-indol-3-yl-bicyclo<2.2.1>hept-2-exo-yl)-benzene-1,3-dimethanimine

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve In chloroform for 48h; Ambient temperature; | 98% |
-
-
626-19-7
Isophthalaldehyde

-
-
46133-07-7
3-((hydroxyimino)methyl)benzaldoxime

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride | 98% |
| With sodium hydroxide; hydroxylamine hydrochloride In ethanol at 0℃; for 1h; | 96% |
| With hydroxylamine hydrochloride; sodium hydroxide In ethanol; water at 0 - 60℃; for 8h; | 85% |
-
-
626-19-7
Isophthalaldehyde

-
-
196929-78-9
(R)-2-methylpropane-2-sulfinamide

| Conditions | Yield |
|---|---|
| With copper(II) sulfate In dichloromethane at 22℃; for 18h; | 98% |

| Conditions | Yield |
|---|---|
| With sulfuric acid-modified polyethyleneglycol-6000 In neat (no solvent) at 70℃; for 0.0333333h; Knoevenagel Condensation; Green chemistry; | 98% |
| With sulfuric acid In acetic acid Heating; | 94% |
m-Phthalaldehyde Chemical Properties
Molecular Structure of Isophthalaldehyde (CAS NO.626-19-7):
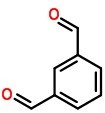
IUPAC Name: benzene-1,3-dicarbaldehyde
Molecular formula: C8H6O2
Molecular Weight: 134.13
EINECS: 210-935-8
Melting point: 87-88 °C(lit.)
Boiling Point: 255.3 °C at 760 mmHg
Flash Point: 94.1 °C
Index of Refraction: 1.622
Molar Refractivity: 39.75 cm3
Molar Volume: 112.7 cm3
Polarizability: 15.76 10-24 cm3
Surface Tension: 48.3 dyne/cm
Density: 1.189 g/cm3
Enthalpy of Vaporization: 49.28 kJ/mol
Vapour Pressure: 0.0164 mmHg at 25°C
Sensitive: Air Sensitive
BRN: 1561038
Stability: Stable. Combustible. Incompatible with strong oxidizing agents.
Appearance: colourless or light yellow crystals
m-Phthalaldehyde Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 100mg/kg (100mg/kg) | U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. Vol. NX#07922, | |
| mouse | LDLo | unreported | 696mg/kg (696mg/kg) | Comptes Rendus Hebdomadaires des Seances, Academie des Sciences. Vol. 246, Pg. 851, 1958. |
m-Phthalaldehyde Safety Profile
Poison by intravenous route. When heated to decomposition it emits acrid smoke and fumes. See also ALDEHYDES.
The Safety Statements information of Isophthalaldehyde (CAS NO.626-19-7):
22: Do not breathe dust
24/25: Avoid contact with skin and eyes
WGK Germany: 3
RTECS: NT1981000
HS Code: 29122900
m-Phthalaldehyde Specification
Isophthalaldehyde , with CAS number of 626-19-7, can be called 1,3-Benzenedicarboxaldehyde (9CI) ; 1,3-benzenedicarboxaldehyde ; Benzol-1,3-dicarbaldehyd . It is a colourless or light yellow crystal. Isophthalaldehyde (CAS NO.626-19-7) is used for pharmaceutical intermediates, fluorescent brighteners, etc.
Related Products
- m-Phthalaldehyde
- 62620-71-7
- 6262-07-3
- 62621-09-4
- 6262-21-1
- 626-22-2
- 62622-28-0
- 6262-27-7
- 6262-30-2
- 626-23-3
- 62624-26-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View