-
Name
2-Bromoanisole
- EINECS 209-425-8
- CAS No. 578-57-4
- Article Data79
- CAS DataBase
- Density 1.443 g/cm3
- Solubility soluble in ethanol and diethyl ether, insoluble in water
- Melting Point 2 °C(lit.)
- Formula C7H7BrO
- Boiling Point 215.999 °C at 760 mmHg
- Molecular Weight 187.036
- Flash Point 96.667 °C
- Transport Information UN 3082 9/PG 3
- Appearance colourless liquid
- Safety 61-24/25
- Risk Codes 51/53
-
Molecular Structure
-
Hazard Symbols
 N,
N, Xi
Xi
- Synonyms Anisole,o-bromo- (6CI,7CI,8CI);1-Bromo-2-methoxybenzene;2-Bromo-1-methoxybenzene;2-Bromomethoxybenzene;2-Methoxyphenylbromide;NSC 6977;o-Anisyl bromide;o-Bromoanisole;o-Bromomethoxybenzene;o-Bromophenyl methyl ether;o-Methoxyphenyl bromide;2-Bromoanisole;
- PSA 9.23000
- LogP 2.45770
Synthetic route
-
-
100-66-3
methoxybenzene

-
A
-
104-92-7
1-bromo-4-methoxy-benzene

-
B
-
578-57-4
2-bromoanisole

-
C
-
21702-84-1
2,4-dibromoanisole

| Conditions | Yield |
|---|---|
| With bromine; tetramethylammonium bromide In liquid sulphur dioxide at -23℃; Rate constant; Product distribution; Thermodynamic data; in H2O at 25 deg C, NaBr, HClO4; ΔH (excit), -ΔS (excit); | A 98.99% B 0.56% C 0.43% |
| With (CH3)4Br In liquid sulphur dioxide at -23℃; Kinetics; Thermodynamic data; Product distribution; ΔH(excit.); ΔS(excit.); | A 98.99% B 0.56% C 0.43% |
| With (CH3)4Br In liquid sulphur dioxide at -23℃; | A 98.99% B 0.56% C 0.43% |


| Conditions | Yield |
|---|---|
| With tert.-butylnitrite; tetrabutylammomium bromide; copper(I) bromide; 10-camphorsulfonic acid In acetonitrile at 20℃; for 24h; Reagent/catalyst; Time; Solvent; | 98% |
| Stage #1: 2-methoxy-phenylamine With tert.-butylnitrite In dichloromethane; water at 0℃; for 0.166667h; Stage #2: With carbon tetrabromide; dimethylglyoxal In dichloromethane; water at 15 - 35℃; for 16h; | 78% |
| With tert.-butylnitrite; tetrabutylammomium bromide; toluene-4-sulfonic acid; copper(ll) bromide In acetonitrile at 20℃; for 23h; | 74% |

| Conditions | Yield |
|---|---|
| With Oxone; potassium bromide In methanol at 20℃; for 1h; | A 97% B 2% |
| With Oxone; potassium bromide In acetonitrile at 20℃; for 24h; | A 84% B 15% |
| With PyHBrCl2 In methanol at 20℃; for 0.0833333h; | A 78% B 18% |

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In acetonitrile at 80℃; for 12h; | 97% |
| With tetrabutylammomium bromide; copper(ll) bromide In water at 100℃; for 8h; Sealed tube; | 82% |
| With 1,10-Phenanthroline; oxygen; potassium bromide; copper(ll) bromide In N,N-dimethyl-formamide at 130℃; for 20h; | 67% |
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; sodium methylate In methanol; water; acetonitrile at 23℃; | 94 % Chromat. |

| Conditions | Yield |
|---|---|
| Stage #1: 2-hydroxybromobenzene With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 1.16667h; Inert atmosphere; Stage #2: methyl iodide In tetrahydrofuran; mineral oil at 20℃; for 19h; Inert atmosphere; Reflux; | 97% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 2.5h; Inert atmosphere; | 96% |
| With potassium hydroxide In CD2Cl2; hexane; acetonitrile | 89% |

| Conditions | Yield |
|---|---|
| With potassium carbonate at 60℃; for 0.3h; Williamson synthesis; | 94% |
| With sodium hydroxide | |
| With potassium hydroxide | |
| With sodium hydroxide for 3h; Heating; | |
| With potassium carbonate In acetone Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With potassium phosphate; tetrabuthylammonium tribromide In acetonitrile at 100℃; for 16h; Reagent/catalyst; | 90% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In N,N-dimethyl-formamide at 120℃; for 4h; | 90% |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In N,N-dimethyl-formamide for 0.0583333h; microwave irradiation; | 84% |
| With leucine intercalated Mg-Al layered double hydorxide at 180℃; for 6h; Autoclave; Green chemistry; chemoselective reaction; | 87 %Chromat. |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 25℃; for 3h; Inert atmosphere; Schlenk technique; | 84% |

-
-
578-57-4
2-bromoanisole

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In N,N-dimethyl-formamide at 20℃; for 4h; | 75% |

| Conditions | Yield |
|---|---|
| With boron trifluoride In diethyl ether for 0.0416667h; microwave irradiation; | 74% |

| Conditions | Yield |
|---|---|
| With butyl magnesium bromide; zirconocene dichloride In tetrahydrofuran for 3h; Ambient temperature; | A 72% B 22% |

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; copper(ll) bromide In water at 100℃; for 8h; Sealed tube; | A 64% B 20% |
| With tetrabutylammomium bromide; copper(ll) bromide In water at 100℃; for 8h; Sealed tube; | A 12% B 26% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; bromine In acetonitrile at 100℃; for 4h; Reagent/catalyst; | A 26% B 56% |

-
-
578-57-4
2-bromoanisole

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; copper In acetonitrile at 20℃; for 0.75h; Substitution; | 55% |
| With tetrabutylammomium bromide In acetonitrile at 60℃; for 0.75h; Substitution; | 53% |
-
-
1232133-61-7
(2,4,6-trimethylphenyl)(2’-methoxyphenyl)iodonium triflate

-
-
578-57-4
2-bromoanisole

| Conditions | Yield |
|---|---|
| With copper(I) bromide In acetonitrile at 80℃; for 2h; | 47% |
-
-
579-75-9
2-Methoxybenzoic acid

-
A
-
578-57-4
2-bromoanisole

-
B
-
21702-84-1
2,4-dibromoanisole

-
C
-
2476-35-9
5-bromo-2-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| With potassium phosphate; 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione In acetonitrile at 100℃; for 4h; | A 40% B 7% C 32% |


| Conditions | Yield |
|---|---|
| With dibromodifluoromethane; ISOPROPYLAMIDE; copper at 150℃; for 8h; Further byproducts given; | 11% |

-
-
60-29-7
diethyl ether

-
-
21702-84-1
2,4-dibromoanisole

-
A
-
104-92-7
1-bromo-4-methoxy-benzene

-
B
-
578-57-4
2-bromoanisole

-
C
-
100-66-3
methoxybenzene


| Conditions | Yield |
|---|---|
| With potassium hydroxide; methyl iodide | |
| With diazomethane; diethyl ether | |
| (methylation); |

| Conditions | Yield |
|---|---|
| With potassium hydroxide |
-
-
38603-09-7
2,6-dibromoanisole

-
-
578-57-4
2-bromoanisole

| Conditions | Yield |
|---|---|
| With water; isopropylmagnesium chloride 1) THF, 40 deg C, 3 h; 2) 0 deg C; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; sodium bromide In methanol for 0.0833333h; Ambient temperature; Yield given; | |
| With N-chloro-succinimide; sodium bromide In methanol for 0.0833333h; Ambient temperature; |

| Conditions | Yield |
|---|---|
| With carbon monoxide at 180℃; |
-
-
74-96-4
ethyl bromide

-
-
60-29-7
diethyl ether

-
-
21702-84-1
2,4-dibromoanisole

-
A
-
104-92-7
1-bromo-4-methoxy-benzene

-
B
-
578-57-4
2-bromoanisole

-
C
-
100-66-3
methoxybenzene

-
-
60333-75-7
O-allyl-2-bromophenol

-
-
74-88-4
methyl iodide

-
A
-
578-57-4
2-bromoanisole

-
B
-
100-66-3
methoxybenzene

| Conditions | Yield |
|---|---|
| Stage #1: O-allyl-2-bromophenol With [2,2]bipyridinyl; tetrabutylammonium tetrafluoroborate; palladium dichloride In N,N-dimethyl-formamide at 20℃; Reduction; Electrochemical reaction; Stage #2: methyl iodide With potassium carbonate In N,N-dimethyl-formamide at 50℃; for 12h; Methylation; |

-
-
5720-06-9
2-Methoxyphenylboronic acid

-
A
-
578-57-4
2-bromoanisole

-
B
-
21702-84-1
2,4-dibromoanisole

-
C
-
89694-45-1
5-bromo-2-methoxyphenylboronic acid

| Conditions | Yield |
|---|---|
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione In water; acetonitrile at 40℃; for 4h; | A 45 % Chromat. B 18 % Chromat. C 34 % Chromat. |

-
-
578-57-4
2-bromoanisole

-
-
21702-84-1
2,4-dibromoanisole

| Conditions | Yield |
|---|---|
| With (CH3)4Br In liquid sulphur dioxide at -23℃; Rate constant; | 100% |
| With (CH3)4Br In liquid sulphur dioxide at -23℃; | 100% |
| With N-Bromosuccinimide; iodine In acetonitrile for 12h; Darkness; | 99% |
| With dihydrogen peroxide; ammonium bromide; acetic acid at 20℃; for 12h; | 54% |
| With bromine; tetramethylammonium bromide In liquid sulphur dioxide at -23℃; Product distribution; Rate constant; |

| Conditions | Yield |
|---|---|
| With di-tert-butyl(4-sulfonatobenzyl)phosphonium; palladium diacetate; sodium carbonate In water at 50℃; for 24h; Solvent; Temperature; Reagent/catalyst; Suzuki Coupling; Inert atmosphere; Glovebox; | 100% |
| With di-tert-butyl(4-sulfonatobenzyl)phosphonium; palladium diacetate; sodium carbonate In water at 50℃; Reagent/catalyst; Solvent; Temperature; Suzuki Coupling; Inert atmosphere; Glovebox; | 100% |
| With sodium carbonate In water at 100℃; Suzuki-Miyaura reaction; | 99.1% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; caesium carbonate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl In toluene for 24h; Buchwald-Hartwig Coupling; Inert atmosphere; Reflux; | 100% |
| With bis[2-(diphenylphosphino)phenyl] ether; sodium t-butanolate; palladium dichloride In toluene at 80℃; for 3h; | 97% |
| With palladium diacetate; bis[2-(diphenylphosphino)phenyl] ether; sodium t-butanolate In toluene at 80℃; for 3h; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromoanisole With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 1h; Inert atmosphere; Stage #2: benzaldehyde In tetrahydrofuran; hexane at -78 - 22℃; for 20h; Inert atmosphere; | 100% |
| Stage #1: 2-bromoanisole With magnesium; lithium chloride In tetrahydrofuran at 50℃; for 0.125h; Flow reactor; Stage #2: benzaldehyde In tetrahydrofuran at 20℃; for 0.333333h; Flow reactor; | 95% |
| Stage #1: 2-bromoanisole With n-butyllithium In tetrahydrofuran; hexane at -78℃; Inert atmosphere; Stage #2: benzaldehyde In tetrahydrofuran; hexane at -78 - 20℃; for 17h; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| With caesium carbonate; diisopropyl(2-tert-butyl)phenoxyphosphine; RhCl(PPh3)3 In toluene for 18h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; palladium dichloride In ethanol; water at 20℃; for 0.25h; Suzuki-Miyaura reaction; | 100% |
| With Pd/C; potassium carbonate In ethanol; water at 100℃; for 3h; Suzuki-Miyaura Coupling; | 95% |
| With cetyltrimethylammonim bromide; sodium hydroxide In water at 25℃; for 1h; Catalytic behavior; Suzuki Coupling; Microwave irradiation; | 94% |
-
-
1663-39-4
tert-Butyl acrylate

-
-
578-57-4
2-bromoanisole

-
-
474097-69-3
tert-butyl (E)-3-(2-methoxyphenyl)prop-2-enoate

| Conditions | Yield |
|---|---|
| With IMes-Pd(dmba)Cl; potassium carbonate In 1-methyl-pyrrolidin-2-one at 140℃; for 18h; Heck-Mizoroki reaction; Inert atmosphere; | 100% |
| With dichlorobis(1,4-dimesityl-1H-1,2,3-triazol-5-ylidene)palladium(II); sodium acetate In N,N-dimethyl acetamide at 150℃; for 8h; Mizoroki-Heck reaction; Inert atmosphere; optical yield given as %de; | 51% |
| With C4H7Cl2Pd(1-)*C27H39N2(1+); potassium carbonate In N,N-dimethyl-formamide at 100℃; for 40h; Heck Reaction; Sealed tube; | 51% |
-
-
578-57-4
2-bromoanisole

-
-
138642-62-3
2-Cyanophenylboronic acid

| Conditions | Yield |
|---|---|
| With sodium tetrachloropalladate(II); 2-(1-(1-phenylethylamino)methyl)pyridine In water; N,N-dimethyl-formamide at 110℃; for 16h; Suzuki-Miyaura coupling; | 100% |
-
-
578-57-4
2-bromoanisole

-
-
29841-69-8
(S,S)-1,2-diphenyl-1,2-diaminoethane

| Conditions | Yield |
|---|---|
| Stage #1: (S,S)-1,2-diphenyl-1,2-diaminoethane With tris-(dibenzylideneacetone)dipalladium(0); 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; sodium t-butanolate In toluene at 20℃; for 1.5h; Inert atmosphere; Schlenk technique; Glovebox; Stage #2: 2-bromoanisole In toluene at 120℃; for 9h; Inert atmosphere; Schlenk technique; Glovebox; | 100% |
-
-
578-57-4
2-bromoanisole

-
-
2043-61-0
cyclohexanecarbaldehyde

-
-
92300-73-7
cyclohexyl (2-methoxyphenyl)methanol

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromoanisole With iodine; magnesium In tetrahydrofuran for 6.16667h; Sealed tube; Inert atmosphere; Reflux; Stage #2: cyclohexanecarbaldehyde In tetrahydrofuran at 20℃; Sealed tube; Inert atmosphere; | 100% |
-
-
106-23-0, 26489-02-1
3,7-dimethyl-oct-6-enal

-
-
578-57-4
2-bromoanisole

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromoanisole With iodine; magnesium In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Stage #2: 3,7-dimethyl-oct-6-enal In tetrahydrofuran at 20℃; for 1.25h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); formaldehyd; caesium carbonate In dimethyl sulfoxide at 80℃; for 12h; | 99% |
| With butyl magnesium bromide; zirconocene dichloride for 12h; Ambient temperature; | 98% |
| With lithium aluminium tetrahydride In 1,2-dimethoxyethane at 35℃; for 4h; ultrasonic acceleration of reduction; | 98% |
-
-
578-57-4
2-bromoanisole

-
-
31600-86-9
2-methoxyphenyllithium

| Conditions | Yield |
|---|---|
| With n-butyllithium In hexane; pentane at 0 - 20℃; for 1h; Inert atmosphere; | 99% |
| With n-butyllithium In hexane; pentane at 0 - 20℃; | 97% |
| With n-butyllithium In hexane; pentane at 20℃; in evacuated, closed vessel; |

| Conditions | Yield |
|---|---|
| With lithium perchlorate at 100℃; for 5h; | 99% |
| With aluminum (III) chloride In dichloromethane at 20 - 40℃; Friedel-Crafts acylation; Inert atmosphere; | 96% |
| With aluminium trichloride In dichloromethane | 68% |

| Conditions | Yield |
|---|---|
| With (R)-1-[(SP)-2-(dicyclohexylphosphino)ferrocenyl]ethyldi-tert-butylphosphine; palladium diacetate; sodium t-butanolate In 1,2-dimethoxyethane at 110℃; for 2h; Inert atmosphere; | 99% |
| With 1,1'-bis-(diphenylphosphino)ferrocene; tris-(dibenzylideneacetone)dipalladium(0); N-ethyl-N,N-diisopropylamine In toluene for 3h; Inert atmosphere; Reflux; | 97% |
| With copper(l) iodide; N,N'-dihexyloxalamide; potassium tert-butylate In 1,4-dioxane at 110℃; for 24h; Sealed tube; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; sodium t-butanolate; ruphos In neat (no solvent) at 110℃; for 12h; Buchwald-Hartwig Coupling; Green chemistry; | 99% |
| With dicyclohexyl(2-methoxy-6-methylbiphenyl-2,-yl)phosphine; potassium tert-butylate; palladium diacetate In 1,4-dioxane at 160℃; for 0.25h; Microwave irradiation; Inert atmosphere; | 80% |
| With tris-(dibenzylideneacetone)dipalladium(0); 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; sodium t-butanolate at 80℃; for 29h; Arylation; | 77% |

| Conditions | Yield |
|---|---|
| With (1-adamantyl)di(tert-butyl) phosphine; triethylamine; bis(dibenzylideneacetone)-palladium(0) In N,N-dimethyl-formamide at 20℃; for 20h; Heck reaction; | 99% |
| With C27H21F3N6OPd; tetrabutylammomium bromide; sodium acetate at 140℃; for 12h; Heck reaction; Inert atmosphere; | 89% |
| With potassium phosphate In N,N-dimethyl-formamide at 130℃; for 15h; Heck Reaction; | 84% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; palladium dichloride In water; N,N-dimethyl-formamide at 20℃; for 0.166667h; Suzuki cross-coupling reaction; | 99% |
| With Ph2P(CH2CH2O)22CH3; triethylamine; palladium dichloride In water at 100℃; for 1h; Suzuki coupling; Inert atmosphere; | 98% |
| With potassium carbonate; Co0.38Fe0.57LaO3Pd0.05 In isopropyl alcohol at 80℃; for 1h; Suzuki reaction; | 89% |
-
-
578-57-4
2-bromoanisole

-
-
172732-52-4
o-cyanophenylboronic acid-1,3-propylene glycol ester

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; potassium phosphate In toluene for 1h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| With (1,3-bis[2,6-bis(diphenylmethyl)-4-methylphenyl]imidazole-2-ylidene)PdCl2(triethylamine); potassium tert-butylate In 1,2-dimethoxyethane at 20℃; for 6h; Buchwald-Hartwig Coupling; Sealed tube; | 99% |
| With potassium tert-butylate In 1,2-dimethoxyethane at 20℃; for 6h; Buchwald-Hartwig Coupling; | 99% |
| With (1,3-bis(2,6-diisopropylphenyl)-3,4,5,6-tetrahydropyrimidin-2-ylidene)Pd(cinnamyl, 3-phenylallyl)Cl; sodium t-butanolate In neat (no solvent) at 110℃; for 12h; Buchwald-Hartwig Coupling; Inert atmosphere; Green chemistry; | 99% |
-
-
67-56-1
methanol

-
-
578-57-4
2-bromoanisole

-
-
201230-82-2
carbon monoxide

-
-
606-45-1
2-methoxybenzoic acid methyl ester

| Conditions | Yield |
|---|---|
| With dichloro[2,2'-bis(diphenylphosphino)-1,1'-binaphthyl]palladium(II); triethylamine at 100℃; under 2585.74 Torr; for 16h; | 99% |

-
-
3612-20-2
1-phenylmethyl-4-piperidone

-
-
578-57-4
2-bromoanisole

-
-
130305-58-7
1-benzyl-4-(2-methoxyphenyl)piperidin-4-ol

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromoanisole With iodine; magnesium In tetrahydrofuran Grignard reaction; Inert atmosphere; Reflux; Stage #2: 1-phenylmethyl-4-piperidone In tetrahydrofuran at 20℃; for 3h; Grignard reaction; Inert atmosphere; Cooling with ice; | 99% |
| Stage #1: 2-bromoanisole With n-butyllithium In tetrahydrofuran; hexane at -70 - 1℃; Stage #2: 1-phenylmethyl-4-piperidone In tetrahydrofuran at 20℃; | 23.1% |
-
-
578-57-4
2-bromoanisole

-
-
156275-96-6
triisopropylsilanethiol

| Conditions | Yield |
|---|---|
| With (R)-(-)-1-[(SP)-2-(dicyclohexylphosphino)ferrocenyl]ethyldi-tert-butylphosphine; palladium diacetate; lithium hexamethyldisilazane In toluene at 110℃; Inert atmosphere; | 99% |
| With caesium carbonate; triphenylphosphine; palladium diacetate In toluene at 100℃; for 16h; | 33% |

| Conditions | Yield |
|---|---|
| With (1,3-bis(2,6-diisopropylphenyl)-3,4,5,6-tetrahydropyrimidin-2-ylidene)Pd(cinnamyl, 3-phenylallyl)Cl; sodium t-butanolate In neat (no solvent) at 110℃; for 12h; Buchwald-Hartwig Coupling; Inert atmosphere; Green chemistry; | 99% |
| With potassium tert-butylate; (ϖ-allyl)(diphosphinecyclobutene)palladium at 20℃; for 12h; | 97% |
| With potassium phosphate; copper(l) iodide In diethylene glycol at 70℃; for 14h; Sealed tube; | 93% |
-
-
578-57-4
2-bromoanisole

-
-
363-72-4
Pentafluorobenzene

-
-
15945-35-4
2,3,4,5,6-pentafluoro-2′-methoxy-1,1′-biphenyl

| Conditions | Yield |
|---|---|
| With dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; palladium diacetate; potassium carbonate In Isopropyl acetate at 80℃; for 12h; | 99% |
| With potassium phosphate; chloro[(tri-tert-butylphosphine)-2-(2-aminobiphenyl)]palladium (II); Trimethylacetic acid In N,N-dimethyl acetamide at 100℃; Glovebox; | 96% |
| With potassium phosphate; chloro[(tri-tert-butylphosphine)-2-(2-aminobiphenyl)] palladium(II); Trimethylacetic acid In N,N-dimethyl acetamide at 80℃; for 2 - 4h; | 96% |

| Conditions | Yield |
|---|---|
| With sodium methylate; nickel diacetate In 1,4-dioxane at 110℃; | 99% |
| With palladium diacetate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; trichlorophosphate at 165℃; for 24h; Inert atmosphere; | 67% |

| Conditions | Yield |
|---|---|
| With caesium carbonate; copper(I) oxide In N,N-dimethyl-formamide at 110℃; for 18h; | 99% |
| With copper diacetate; sodium hydroxide; 3-(diphenylphosphino)propionic acid In 1,4-dioxane at 120℃; for 36h; Sealed tube; | 51% |
2-Bromoanisole Consensus Reports
2-Bromoanisole Specification
The 2-Bromoanisole, with the CAS registry number 578-57-4, is also known as 2-Bromo-1-methoxybenzene. It belongs to the product categories of Aromatic Halides(substituted); Anisole; Miscellaneous; Anisoles, Alkyloxy Compounds & Phenylacetates; Bromine Compounds. Its EINECS number is 209-425-8. This chemical's molecular formula is C7H7BrO and molecular weight is 187.03. What's more, its systematic name is 1-Bromo-2-methoxybenzene. It is used in organic synthesis. This chemical should be sealed and stored in a cool and dry place. Moreover, it should be protected from oxides, light and moisture.
Physical properties of 2-Bromoanisole are: (1)ACD/LogP: 2.705; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.71 ; (4)ACD/LogD (pH 7.4): 2.71; (5)ACD/BCF (pH 5.5): 67.00; (6)ACD/BCF (pH 7.4): 67.00; (7)ACD/KOC (pH 5.5): 705.86; (8)ACD/KOC (pH 7.4): 705.86; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 9.23 Å2; (13)Index of Refraction: 1.539; (14)Molar Refractivity: 40.622 cm3; (15)Molar Volume: 129.621 cm3; (16)Polarizability: 16.104×10-24cm3; (17)Surface Tension: 34.6 dyne/cm; (18)Density: 1.443 g/cm3; (19)Flash Point: 96.667 °C; (20)Enthalpy of Vaporization: 43.392 kJ/mol; (21)Boiling Point: 215.999 °C at 760 mmHg; (22)Vapour Pressure: 0.2 mmHg at 25°C.
Preparation of 2-Bromoanisole: this chemical can be prepared by methoxybenzene at the temperature of 20 °C. This reaction will need reagents Oxone, potassium bromide and solvent methanol with the reaction time of 1 hour. The yield is about 97%.

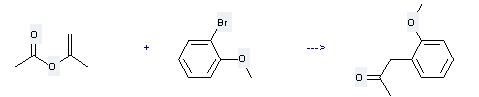
Uses of 2-Bromoanisole: it can be used to produce (2-methoxy-phenyl)-acetone at the temperature of 100 °C. It will need regent tributyltin methoxide and solvent toluene with the reaction time of 5 hours. This reaction will also need catalyst dichlorobis(tri-o-tolylphosphine)palladium. The yield is about 90%.
When you are using this chemical, please be cautious about it as the following:
This chemical is toxic to aquatic organisms as it may cause long-term adverse effects in the aquatic environment. You should avoid releasing it to the environment, and you need to refer to special instructions/safety data sheet. When using it, you must avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
(1)SMILES: COc1ccccc1Br
(2)Std. InChI: InChI=1S/C7H7BrO/c1-9-7-5-3-2-4-6(7)8/h2-5H,1H3
(3)Std. InChIKey: HTDQSWDEWGSAMN-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 1544mg/kg (1544mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 44(12), Pg. 19, 1979. | |
| mouse | LD50 | oral | 2466mg/kg (2466mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 44(12), Pg. 19, 1979. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View