-
Name
Benzene
- EINECS 200-753-7
- CAS No. 71-43-2
- Article Data3748
- CAS DataBase
- Density 0.873 g/cm3
- Solubility 0.18 g/100 mL in water
- Melting Point 5.5 °C
- Formula C6H6
- Boiling Point 78.834 °C at 760 mmHg
- Molecular Weight 78.1136
- Flash Point -11 °C
- Transport Information UN 1114 3/PG 2
- Appearance clear colorless liquid with a petroleum-like odor
- Safety 53-45-36/37
- Risk Codes 45-46-11-36/38-48/23/24/25-65-39/23/24/25-23/24/25
-
Molecular Structure
-
Hazard Symbols
 F,
F, T
T
- Synonyms 1,3,5-Cyclohexatriene;Benzol;Benzole;Coal naphtha;Cyclohexatriene;NSC 67315;Phene;Phenylhydride;Pyrobenzol;Pyrobenzole;[6]Annulene;crude benzene;
- PSA 0.00000
- LogP 1.68660
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium hydroxide; ethanol; hydrogen; PdCl2-poly(N-vinyl-2-pyrrolidone); palladium dichloride at 65℃; under 760 Torr; for 2h; | 100% |
| With 9-borabicyclo[3.3.1]nonane dimer; triphenylstannane In toluene at 0℃; | 100% |
| With TiCpCl2; diisobutylaluminium hydride In 1,4-dioxane at 80℃; for 4h; Product distribution; further reagents(Zr, Hf complexes), solvents, and temperatures; further halobenzenes, alkyl, alkenyl and cyclopropyl halides; | 100% |

| Conditions | Yield |
|---|---|
| With tetrabutoxytitanium; diisobutylaluminium hydride In diethyl ether for 6h; Heating; | 100% |
| With sodium hydroxide; ethanol; PdCl2-poly(N-vinyl-2-pyrrolidone); palladium dichloride at 65℃; under 760 Torr; for 1h; | 100% |
| With potassium tert-butylate; benzaldehyde In N,N-dimethyl-formamide at 90℃; for 1h; Schlenk technique; Inert atmosphere; | 99% |
-
-
1165952-92-0
cyclohexa-1,4-diene

-
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| With [Mn(IV)(O)(BQCN)(H2O)](2+) In water; acetonitrile at 0℃; Kinetics; Concentration; Inert atmosphere; | 100% |
| With Fe2(OH)0.6(2,5-dioxido-1,4-benzenedicarboxylate) at 24℃; | 100% |
| With oxygen; 2,3-dicyano-5,6-dichloro-p-benzoquinone; sodium nitrite In toluene at 120℃; under 9750.98 Torr; for 8h; | 99% |
-
-
1165952-91-9
cyclohexa-1,3-diene

-
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; polystyrene-bound phenylselenic acid In tetrachloromethane for 12h; Product distribution; Heating; var. aromatic species; var. reaction times; | 100% |
| With iron(III) trifluoromethanesulfonate; C65H77N5O4S2 In 1,2-dichloro-ethane at 75℃; under 760.051 Torr; for 8h; Inert atmosphere; | 78% |
| With [[HC-(CMeNC6H3(iPr)2)2]NiIII(O2)CuIII(N(indane)(2-pyridyl(ethylamine))2)](OTf) In dichloromethane at -60℃; Kinetics; Reagent/catalyst; Inert atmosphere; | 49% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; ethanol; hydrogen; PdCl2-poly(N-vinyl-2-pyrrolidone); palladium dichloride at 65℃; under 760 Torr; for 2h; Product distribution; effect of bases and solvents on the hydrodechlorination; | 100% |
| With potassium hydroxide; hydrogen; palladium on activated charcoal; Aliquat 336 In 2,2,4-trimethylpentane at 50℃; for 4.5h; | 100% |
| With sodium hydroxide; ethanol; hydrogen; PdCl2-poly(N-vinyl-2-pyrrolidone); palladium dichloride at 65℃; under 760 Torr; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; hydrogen; palladium on activated charcoal; Aliquat 336 In water at 50℃; for 0.833333h; | 100% |
| With di-tert-butyl peroxide; caesium carbonate; isopropyl alcohol at 120℃; for 3h; Inert atmosphere; | 93 %Chromat. |
| With ammonium formate In methanol at 20℃; for 16h; |
-
-
3968-30-7
5-cyclopentylidene-2,2-dimethyl-1,3-dioxane-4,6-dione

-
A
-
2873-50-9
butatriene

-
B
-
74-85-1
ethene

-
C
-
124-38-9
carbon dioxide

-
D
-
1165952-91-9
cyclohexa-1,3-diene

-
E
-
67-64-1
acetone

-
F
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| With variation of temp. at 550℃; Product distribution; | A 4% B 11.9% C 100% D 39.2% E 101.9 % F 3.3% |


| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In water at 50℃; for 0.25h; | 100% |
| With sodium hydroxide; ethanol; hydrogen; PdCl2-poly(N-vinyl-2-pyrrolidone); palladium dichloride at 65℃; under 760 Torr; for 6h; | 100% |
| With Ni[1,10-phenanthroline]2(PF6)2; water; zinc at 70℃; for 7h; Ionic liquid; | 51.2% |
-
-
1113-69-5
1,1-dicyano-2,2-bis(trifluoromethyl)ethene

-
A
-
82947-39-5
2,2-Bis(trifluoromethyl)ethane-1,1-dicarbonitrile

-
B
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| With cyclohexa-1,4-diene In chloroform-d1 Mechanism; Ambient temperature; or 1,3-cyclohexadiene, 2,5-dihydrofuran; | A 100% B 97% |
| With cyclohexa-1,4-diene In chloroform-d1 Ambient temperature; also with 1,3-cyclohexadiene, 2,5-dihydrofuran; | A 100% B 97% |
-
-
97111-31-4, 97111-47-2
C22H17ClN6O

-
A
-
97111-56-3
4-Chloro-1-(4-methoxy-phenyl)-[1,2,4]triazolo[4,3-a]quinoxaline

-
B
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| In chloroform-d1 at 60℃; for 0.0333333h; Product distribution; Thermodynamic data; Rate constant; other temperature, time; | A 100% B 80% |
-
-
86379-23-9
5-(2-Chloro-phenoxy)-1-phenyl-1H-tetrazole

-
A
-
5097-82-5
1-phenyl-5-hydroxytetrazole

-
B
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| With cyclohexene; palladium on activated charcoal In ethanol; water; benzene for 4.16667h; Heating; | A n/a B 100% |

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal; Aliquat 336 In water at 50℃; for 0.25h; | 100% |
| With cyclohexane; CeCl3/C; palladium dichloride at 300℃; Product distribution; Further Variations:; Reagents; Temperatures; | 99.9% |
| With Ni[1,10-phenanthroline]2(PF6)2; water; zinc at 70℃; for 7h; Ionic liquid; | 58.2% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; ethanol; hydrogen; PdCl2-poly(N-vinyl-2-pyrrolidone); palladium dichloride at 65℃; under 760 Torr; for 2h; | 100% |
| With Ni[1,10-phenanthroline]2(PF6)2; water; zinc at 70℃; for 7h; Ionic liquid; | 46% |
| With Ni-Al clusters In tetrahydrofuran for 1.5h; Dehalogenation; reduction; hydrogenolysis; dechlorination; Heating; |

| Conditions | Yield |
|---|---|
| In (2)H8-toluene at 120℃; for 30h; | A 100% B n/a |

-
-
1483-73-4
diphenyliodonium bromide

-
A
-
591-50-4
iodobenzene

-
B
-
92-52-4
biphenyl

-
D
-
1192-89-8
bromo(phenyl)mercury

-
E
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| In acetonitrile Kinetics; byproducts: C6H5Br; at different temp. between 20-70°C; UV; yields for 45°C; | A 100% B 6-7 C n/a D n/a E 1.4% |

-
-
1483-72-3
diphenyliodonium chloride

-
A
-
591-50-4
iodobenzene

-
C
-
92-52-4
biphenyl

-
D
-
100-56-1
phenylmercury(II) chloride

-
E
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| In acetonitrile Kinetics; at different temp. between 20-70°C; UV; yields for 45°C; | A 100% B 10-11 C 4-5 D 89-92 E 1.4% |


-
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper

-
A
-
462-06-6
fluorobenzene

-
B
-
591-50-4
iodobenzene

-
C
-
92-52-4
biphenyl

-
D
-
14708-11-3
copper(I) tetrafluoroborate

-
E
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| In acetonitrile Kinetics; stirred at 20-70°C; GLC; | A <1 B 100% C 95% D 99% E 5% |


| Conditions | Yield |
|---|---|
| In chlorobenzene byproducts: benzene; stirring soln. under Ar for 48 h at 110°C; distn. of solvent, G.C.anal. of benzene; | A 100% B 100% |
-
-
21373-88-6, 16920-54-0
(PPh3)3CoH(N2)

-
-
1579-72-2
2,2,2-trifluoroethyl benzoate

-
A
-
99668-73-2
(trifluoroethoxo)tris(triphenylphosphine)cobalt(I)

-
B
-
120-51-4
benzoic acid benzyl ester

-
C
-
7727-37-9
nitrogen

-
D
-
1333-74-0
hydrogen

-
E
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| In toluene PhCOOCH2CF3 added to toluene soln. of CoH(N2)(PPh3)3, evacuated, stirred at 20°C for 2 days; | A n/a B 28% C 100% D 17% E 32% |


| Conditions | Yield |
|---|---|
| With isopropyl alcohol at 150℃; under 7500.75 Torr; for 12h; Inert atmosphere; Autoclave; | A 25.1% B 100% C 74.9% |
| With isopropyl alcohol at 160℃; for 15h; Autoclave; Inert atmosphere; | |
| With isopropyl alcohol at 150℃; for 10h; Catalytic behavior; Reagent/catalyst; Temperature; Sealed tube; | A 24.6 %Chromat. B 47.8 %Chromat. C 24.3 %Chromat. |
| With isopropyl alcohol at 150℃; for 6h; Temperature; Sealed tube; | A 15.2 %Chromat. B 17.7 %Chromat. C 5.8 %Chromat. |

-
-
88242-78-8
phenyl(p-carboran-2-yl)iodonium tetrafluoroborate

-
A
-
591-50-4
iodobenzene

-
B
-
22784-33-4
2-iodo-closo-1,12-dodecaborane

-
C
-
22762-43-2
2-fluoro-p-caborane

-
D
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| In dichloromethane; water 25°C, 0.6 h; | A 99% B 0% C 99.5% D <0.5 |

-
-
34557-54-5
methane

-
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| With hydrogen; molybdenum and barium-containing crystalline metallosilicate catalyst [Mo-B] at 200 - 700℃; Product distribution / selectivity; | 99% |
| chromium(VI) oxide; HZHM (pentasil); platinum at 750℃; Product distribution; | 14% |
| molybdenum for 2h; Product distribution / selectivity; Inert atmosphere; | 12.4% |

| Conditions | Yield |
|---|---|
| at 930℃; under 0.1 Torr; Product distribution; Mechanism; detected by 13C nmr; | A 99% B 1% |

-
-
88242-78-8
phenyl(p-carboran-2-yl)iodonium tetrafluoroborate

-
A
-
591-50-4
iodobenzene

-
B
-
22784-33-4
2-iodo-closo-1,12-dodecaborane

-
C
-
22762-43-2
2-fluoro-p-caborane

-
D
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| In dichloromethane; water at 25°C, 0.5 h; | A 95% B <1 C 99% D <0.5 |


-
A
-
92-52-4
biphenyl

-
B
-
139-66-2
diphenyl sulfide

-
C
-
603-36-1
triphenylantimony

-
D
-
882-33-7
diphenyldisulfane

-
E
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| In neat (no solvent) thermal decompn. of Sb compd. in a sealed tube on heating to 140°C for 3 h; not isolated, detected by GLC and TLC; | A 21% B 69% C 99% D 30% E 6% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide at 24.84℃; under 760.051 Torr; for 12h; Inert atmosphere; UV-irradiation; Sealed tube; | 99% |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In 1,2-dimethoxyethane at 35℃; for 4h; ultrasonic acceleration of reduction; | A 98% B n/a |
| With lithium aluminium tetrahydride; Tridecane In diethyl ether at 0℃; for 0.25h; Irradiation; Title compound not separated from byproducts; | A 77 % Chromat. B 18 % Chromat. |
| With Amberlite IRA-400; borohydride form; nickel diacetate In methanol at 20℃; for 3h; Reduction; | A 56 % Chromat. B 19 % Chromat. |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; PEG-6000 at 150℃; for 6h; | A 98% B n/a |


| Conditions | Yield |
|---|---|
| With sodium methylate; bis-triphenylphosphine-palladium(II) chloride In dichloromethane at 40℃; under 11250.9 Torr; for 4h; | A 98% B 1% |


| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In 1,2-dimethoxyethane at 35℃; for 4h; ultrasonic acceleration of reduction; | A 98% B n/a |

| Conditions | Yield |
|---|---|
| With aluminium trichloride for 19.5h; Heating; | 100% |
| Friedel-Crafts reaction; | 90% |
| iron(III) chloride at 109℃; for 0.458333h; Product distribution / selectivity; Irradiation; | 83% |

| Conditions | Yield |
|---|---|
| With Nafion-H at 150℃; for 0.5h; Microwave irradiation; | 100% |
| With boron trifluoride monohydrate at 50℃; for 2h; Sealed tube; | 98% |
| With trifluorormethanesulfonic acid at 50℃; for 0.583333h; | 96% |

| Conditions | Yield |
|---|---|
| With Anthyllis vulneraria/Noccaea caerulescens extracts supported in montmorillonite K10 at 25℃; for 3h; Friedel Crafts alkylation; regioselective reaction; | 100% |
| lithium tetrakis(pentafluorophenyl)borate for 8h; Friedel-Crafts benzylation; Heating; | 96% |
| With carbon monoxide at 130℃; under 7600.51 Torr; for 10h; Friedel-Crafts alkylation; | 95% |

| Conditions | Yield |
|---|---|
| lithium tetrakis(pentafluorophenyl)borate for 8h; Friedel-Crafts benzylation; Heating; | 100% |
| With phosphonic Acid at 150℃; for 36h; Inert atmosphere; | 56% |
| aluminium trichloride In nitromethane at 20℃; Rate constant; | |
| With aluminium trichloride |

| Conditions | Yield |
|---|---|
| With zinc(II) chloride In dichloromethane at 20℃; for 24h; | 100% |
| With aluminium trichloride | 58% |
| With aluminum (III) chloride at 50 - 60℃; for 2h; | 50% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In dichloromethane at 0 - 20℃; Friedel-Crafts Acylation; Inert atmosphere; | 100% |
| With iron(III) oxide at 20℃; for 0.166667h; Friedel Crafts acylation; regioselective reaction; | 98% |
| With zinc at 60 - 62℃; for 0.00833333h; Friedel-Crafts acylation; microwave irradiation; | 95% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In dichloromethane at 0 - 20℃; Inert atmosphere; | 100% |
| aluminium trichloride; nitromethane In carbon disulfide for 2h; Product distribution; Mechanism; Ambient temperature; further Friedel-Crafts catalysts, further arenes, further haloacid chlorides; variation of temperature and time; | 88% |
| aluminium trichloride; nitromethane In carbon disulfide for 2h; Ambient temperature; | 88% |

| Conditions | Yield |
|---|---|
| With aluminium trichloride In dichloromethane at 20 - 40℃; for 2.5h; | 100% |
| With aluminum (III) chloride at 20 - 30℃; for 3h; Friedel-Crafts Acylation; | 90.1% |
| With carbon disulfide; aluminium trichloride | |
| With aluminium trichloride |

| Conditions | Yield |
|---|---|
| With carbon dioxide; bromine at 40℃; under 187519 Torr; for 2h; Supercritical conditions; Green chemistry; | 100% |
| With lead(II,IV) oxide; trifluoroacetic acid; potassium bromide at 20℃; Product distribution; reagents ratio; | 96% |
| With gold(III) chloride; N-Bromosuccinimide In 1,2-dichloro-ethane at 80℃; for 24h; Inert atmosphere; regioselective reaction; | 94% |

| Conditions | Yield |
|---|---|
| With cobalt(III) acetate; trifluoroacetic acid; potassium iodide In water at 25℃; for 0.00333333h; Mn(OAc)3 or (NH4)2Ce(SO4)3 instead Co(OAc)3; object of study: oxidative iodination promoted by Co(III), Ni(III) or Ce(IV); | 100% |
| With cobalt(III) acetate; trifluoroacetic acid In water at 25℃; for 0.00333333h; | 100% |
| With iodine; silver trifluoromethanesulfonate In dichloromethane at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| With hydrogen; [(norbornadiene)rhodium(I)chloride]2; polydiacetylene In n-heptane at 30℃; under 60800 Torr; for 0.9h; Product distribution; various aromatic compounds and other catalyst also investigated; | 100% |
| With hydrogen; Ni-Tc on γ-Al2O3 at 175 - 250℃; under 760 Torr; Product distribution; dependence of catalytic activity on the reduction temperature; enhanced activity of bimetallic catalysts; | 100% |
| With hydrogen; decacarbonyldirhenium(0) at 230℃; under 75005.9 Torr; for 0.25h; | 100% |

| Conditions | Yield |
|---|---|
| With water-d2; [Ru(2,6-bis((di-t-Bu-phosphino)methyl)pyridine)(η2-H2)H2] In cyclohexane at 50℃; for 72h; | 100% |
| With CD5(1+) In gas at 57.9℃; Rate constant; experiment conditions: NBS pulsed ion cyclotron resonance; | |
| With C(2)H3CN(2)H(1+) In gas at 57.9℃; Rate constant; Mechanism; Thermodynamic data; experiment conditions: NBS pulsed ion cyclotron resonance; probability of a reactive H/D exchange encounter for the deuteronated ion as a function of the Gibbs free-energy change of the (endoergic) deuteron transfer reaction; further reagents; |
-
-
71-43-2
benzene

-
-
608-73-1, 119911-70-5
1,2,3,4,5,6-hexachlorocyclohexane

| Conditions | Yield |
|---|---|
| With chlorine for 48h; Irradiation; | 100% |
| With chlorine Rate constant; Irradiation; | |
| With chlorine im Sonnenlicht; α-benzene hexachloride; |

| Conditions | Yield |
|---|---|
| With tris-(2-chloro-ethyl)-amine; trifluorormethanesulfonic acid; trifluoroacetic acid In chloroform at 40℃; for 12h; Product distribution; Mechanism; various acids, various substrates; | 100% |
| With trifluorormethanesulfonic acid; trimethylsilylazide at 55℃; for 0.833333h; Product distribution; Mechanism; other arenes or substituted arenes; var. temperatures and time; | 95% |
| With trifluorormethanesulfonic acid; trimethylsilylazide In chloroform at 90℃; under 5250.53 Torr; for 0.0466667h; Flow reactor; | 86% |

| Conditions | Yield |
|---|---|
| rhodium(II) trifluoroacetate dimer at 22℃; for 2h; Product distribution; other aromatic reaction partners, other diazo esters; | 100% |
| rhodium(II) trifluoroacetate dimer at 22℃; for 2h; Product distribution; var. rhodium(II) salt; | 100% |
| rhodium(II) trifluoroacetate dimer at 22℃; for 2h; | 100% |
| Heating; |
-
-
623-73-4
diazoacetic acid ethyl ester

-
-
71-43-2
benzene

-
-
27332-37-2
ethyl cyclohepta-2,4,6-trienecarboxylate

| Conditions | Yield |
|---|---|
| With Ag3(μ2-(3,5-(CF3)2PyrPy))3 In dichloromethane; benzene at 25℃; for 2h; Catalytic behavior; Time; Solvent; Buchner Ring Enlargement; Darkness; | 100% |
| rhodium(II) trifluoroacetate dimer at 22℃; for 2h; | 98% |
| With C28H6Au2Cu2F24N2 In cyclohexane for 12h; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid at 20℃; for 3h; | 100% |
| With aluminium trichloride at 60℃; for 2h; Yield given; |

| Conditions | Yield |
|---|---|
| for 1h; Irradiation; | 100% |
| In acetonitrile for 1h; Irradiation; | 98% |
| at 20℃; Quantum yield; Irradiation; | |
| With benzophenone at 20℃; Quantum yield; Further Variations:; Reagents; Irradiation; |
Benzene Specification
Benzene, also kanow as (6)Annulene, is clear colorless, highly flammable liquid with a petroleum-like odor. It evaporates into the air very quickly. Benzene is a natural constituent of crude oil, and is one of the most basic petrochemicals. It is widely used as solvent and precursor in a range of products. By substitution, addition reaction and benzene ring rupture reaction, many important chemical intermediates can be derived.
Benzene is insoluble in water. In addition to glycerol, ethylene glycol, diethylene glycol, 1,4 - butanediol and other polyols, Benzene can be miscible with alcohol, chloroform, ether, carbon tetrachloride, carbon disulfide, glacial acetic acid, acetone, toluene, xylene, aliphatic hydrocarbon and most organic solvents. With the exception of slightly dissolved iodine and sulfur, the inorganic substance is insoluble in benzene.
Preparation: The production and supply of benzene in different countries and regions are not the same. The United States is mainly get the benzene from the restructuring gasoline. The Western Europe is mainly from pyrolysis gasoline. And China is mainly from the restructuring gasoline and coking by-product.
1. Coking by-product: There is part of benzene in the high temperature tar of coking by-product. After some treamtent, benzene is obtained.
2. Pyrolysis gasoline: Benzene The pyrolysis gasoline generally contains about 40% -70% aromatic hydrocarbon. About 37% of aromatic hydrocarbon is benzene, about 14% is toluene, and about 5% is xylene. Benzene can be extracted by hydrogenation and dealkylation.
3. Benzene also can be prepared by toluene from hydrodealkylation. In this hydrogen-intensive process, toluene is mixed with hydrogen. Then in the presence of catalysts Chromium, Molybdenum or Platinum oxide, the reaction happens at 500–600 °C and 40–60 atm pressure. A typical reaction yield exceeds 95 %.
C6H5CH3 + H2 → C6H6 + CH4
Uses: 1. Benzene is used as important raw material of synthetic dye, synthetic rubbers, synthetic resin, synthetic fiber, synthetic grain, plastics, pharmaceuticals, pesticides, photographic film and petrochemical products. Benzene has good solubility, so it is widely used as adhesive and industrial solvent such as lacquer thinner of varnish and nitrocellulose, paint remover, lubricant, grease, wax, celluloid, resin, leather.
2. Benzene is also used as standard sample in the measurment of refractive index. And it can be used as solvent and cleaning agent in precision optical instruments, electronics and so on.
3. It is used as solvent of cosmetics, paint, rubber, glue, etc. Today, benzene is used mainly as an intermediate to make other chemicals. For example: it can react with azidobenzene to get diphenylamine. This reaction needs reagent at temperature of 55 °C. The reaction time is 1 hours. The yield is 82%.
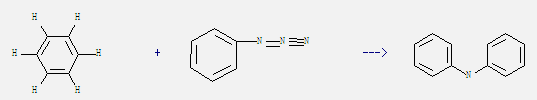
Safty: Benzene is toxic by inhalation, in contact with skin and if swallowed. Moreover, it is harmful that may cause lung damage if swallowed. In addition, Benzene has danger of very serious irreversible effects and serious damage to health by prolonged exposure that may cause heritable genetic damage. If you want to contact this product, you must wear suitable protective clothing and gloves. In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) Please avoid exposure - obtain special instructions before use.
Structure Descriptors:
1. Smiles:c1ccccc1
2. InChI:InChI=1/C6H6/c1-2-4-6-5-3-1/h1-6H
Toxicity:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LCLo | inhalation | 170000mg/m3 (170000mg/m3) | "Handbook of Toxicology," 4 vols., Philadelphia, W.B. Saunders Co., 1956-59Vol. 1, Pg. 324, 1955. | |
| dog | LCLo | inhalation | 146000mg/m3 (146000mg/m3) | "Handbook of Toxicology," 4 vols., Philadelphia, W.B. Saunders Co., 1956-59Vol. 1, Pg. 324, 1955. | |
| dog | LDLo | oral | 2gm/kg (2000mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1313, 1935. | |
| frog | LDLo | subcutaneous | 1400mg/kg (1400mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1313, 1935. | |
| guinea pig | LD50 | skin | > 9400uL/kg (9.4mL/kg) | Toxicology and Applied Pharmacology. Vol. 7, Pg. 559, 1965. | |
| guinea pig | LDLo | intraperitoneal | 527mg/kg (527mg/kg) | "Handbook of Toxicology," 4 vols., Philadelphia, W.B. Saunders Co., 1956-59Vol. 1, Pg. 42, 1955. | |
| human | LCLo | inhalation | 65mg/m3/5Y (65mg/m3) | BLOOD: OTHER CHANGES | Archiv fuer Geschwulstforschung. Vol. 44, Pg. 145, 1974. |
| human | LCLo | inhalation | 2pph/5M (20000ppm) | Tabulae Biologicae. Vol. 3, Pg. 231, 1933. | |
| human | TCLo | inhalation | 100ppm (100ppm) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" GASTROINTESTINAL: NAUSEA OR VOMITING | Industrial Medicine. Vol. 17, Pg. 199, 1948. |
| mammal (species unspecified) | LCLo | inhalation | 20000ppm/5M (20000ppm) | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 138, Pg. 65, 1928. | |
| mammal (species unspecified) | LD50 | oral | 5700mg/kg (5700mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 39(4), Pg. 86, 1974. | |
| mammal (species unspecified) | LDLo | intraperitoneal | 1500mg/kg (1500mg/kg) | PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE) BEHAVIORAL: IRRITABILITY BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | American Journal of Hygiene. Vol. 7, Pg. 276, 1927. |
| man | LDLo | oral | 50mg/kg (50mg/kg) | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 22, Pg. 883, 1980. | |
| man | LDLo | unreported | 194mg/kg (194mg/kg) | "Poisoning; Toxicology, Symptoms, Treatments," 2nd ed., Arena, J.M., Springfield, IL, C.C. Thomas, 1970Vol. 2, Pg. 73, 1970. | |
| man | TCLo | inhalation | 150ppm/1Y-I (150ppm) | BLOOD: OTHER CHANGES | Blut. Vol. 28, Pg. 293, 1974. |
| mouse | LC50 | inhalation | 9980ppm (9980ppm) | BEHAVIORAL: GENERAL ANESTHETIC BEHAVIORAL: MUSCLE WEAKNESS LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Journal of Industrial Hygiene and Toxicology. Vol. 25, Pg. 366, 1943. |
| mouse | LD50 | intraperitoneal | 340mg/kg (340mg/kg) | Annals of the New York Academy of Sciences. Vol. 243, Pg. 104, 1975. | |
| mouse | LD50 | oral | 4700mg/kg (4700mg/kg) | Hygiene and Sanitation Vol. 32(3), Pg. 349, 1967. | |
| mouse | LD50 | skin | 48mg/kg (48mg/kg) | Raw Material Data Handbook, Vol.1: Organic Solvents, 1974. Vol. 1, Pg. 5, 1974. | |
| rabbit | LCLo | inhalation | 45000ppm/30M (45000ppm) | Journal of Industrial Hygiene and Toxicology. Vol. 26, Pg. 69, 1944. | |
| rabbit | LD50 | skin | > 9400uL/kg (9.4mL/kg) | Toxicology and Applied Pharmacology. Vol. 7, Pg. 559, 1965. | |
| rabbit | LDLo | intravenous | 88mg/kg (88mg/kg) | PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE GASTROINTESTINAL: GASTRITIS SENSE ORGANS AND SPECIAL SENSES: HEMORRHAGE: EYE | Journal of Toxicology and Environmental Health. Vol. (Suppl, |
| rat | LC50 | inhalation | 10000ppm/7H (10000ppm) | "Toxicology and Biochemistry of Aromatic Hydrocarbons," Gerarde, H., New York, Elsevier, 1960Vol. -, Pg. 113, 1960. | |
| rat | LD50 | intraperitoneal | 1100ug/kg (1.1mg/kg) | Acta Physiologica Polonica. Vol. 12, Pg. 173, 1961. | |
| rat | LD50 | oral | 930mg/kg (930mg/kg) | BEHAVIORAL: TREMOR BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Toxicology and Applied Pharmacology. Vol. 7, Pg. 767, 1965. |
Related Products
- Benzene
- Benzene, (10-bromodecyl)-
- Benzene,1,1',1'',1'''-(oxydimethylidyne)tetrakis-
- Benzene,1,1',1'',1'''-silanetetrayltetrakis-
- Benzene,1,1',1'',1'''-silanetetrayltetrakis[4-methyl-
- Benzene,1,1'-[1,2-bis(methylene)-1,2-ethanediyl]bis-
- Benzene,1,1'-(1,2-cyclopropanediyl)bis-
- Benzene,1,1'-(1,2-ethanediyl)bis(2,4-dinitro-)
- Benzene,1,1'-(1,2-ethanediyl)bis[2-methyl-
- Benzene,1,1'-(1,2-ethanediyl)bis[4-methyl-
- 7143-23-9
- 71436-95-8
- 7143-76-2
- 7143-82-0
- 7144-05-0
- 71440-79-4
- 7144-08-3
- 71441-28-6
- 71441-76-4
- 7144-20-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View