Dayang Chem (Hangzhou) Co.,Ltd.
Dayangchem's R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. DayangChem can provide different quantities
Cas:67843-74-7
Min.Order:1 Kilogram
FOB Price: $3.0
Type:Lab/Research institutions
inquirySimagchem Corporation
Welcome to Simagchem, your partner in China as a premier supply of bulk specialty chemicals for industry and life science. We introduce experienced quality product and exceptional JIT service with instant market intelligence in China to benefit our
Cas:67843-74-7
Min.Order:1 Metric Ton
Negotiable
Type:Manufacturers
inquiryHangzhou Dingyan Chem Co., Ltd
Items Standard Result Assay (Ursolic acid) 98%min 98.22% ----------------------------------------------------------------
Cas:67843-74-7
Min.Order:1 Gram
FOB Price: $100.0 / 500.0
Type:Trading Company
inquiryWuhan Fortuna Chemical Co.,Ltd
High quality (S)-(+)-Epichlorohydrin Cas 67843-74-7 with good price Company profile Wuhan Fortuna Chemical Co.,Ltd established in 2006, is a big integrative chemical enterprise being engaged in Pharmaceutical & its intermediates, Food/Feed a
Cas:67843-74-7
Min.Order:10 Kilogram
FOB Price: $10.0
Type:Trading Company
inquiryHenan Allgreen Chemical Co.,Ltd
high quality Appearance:White or off-white Solid Storage:Sealed, dry, microtherm , avoid light and smell. Package:According to the demand of customer Application:Organic synthesis Transportation:by air or by sea Port:shanghai
Ality Chemical Corporation
The above product is Ality Chemical's strong item with best price, good quality and fast supply. Ality Chemical has been focusing on the research and production of this field for over 14 years. At the same time, we are always committed to providi
Cas:67843-74-7
Min.Order:1
Negotiable
Type:Other
inquiryEnke Pharma-tech Co.,Ltd. (Cangzhou, China )
Cangzhou Enke Pharma Tech Co.,ltd. is located in Cangzhou City, Hebei province ,where is a famous petroleum chemical industry city in China. Enke Pharma a high-tech enterprise ,and we are dedicated to developing and manufacturing new ap
Cas:67843-74-7
Min.Order:1 Kilogram
FOB Price: $5.0
Type:Manufacturers
inquiryChemwill Asia Co., Ltd.
Cas:67843-74-7
Min.Order:1 Metric Ton
FOB Price: $1.0
Type:Manufacturers
inquiryLIDE PHARMACEUTICALS LIMITED
LIDE PHARMACEUTICALS LIMITED is a professional chemicals and APIs leading manufacturer in China. Our core business line covers APIs, Intermediates, Herb extract, etc. We are specialized in chemical synthesis, process development of pharm
Cas:67843-74-7
Min.Order:1 Kilogram
FOB Price: $25.0 / 30.0
Type:Lab/Research institutions
inquiryHenan Tianfu Chemical Co., Ltd.
(S)-(+)-Epichlorohydrin Basic information Product Name: (S)-(+)-Epichlorohydrin Synonyms: G-CHLOROPROPYLENE OXIDE;EPICHLORHYDRIN;EPICHLOROHYDRINE;CHLOROMETHYLOXIRANE;2,3-EPOXYPROPYL CHLO
Cas:67843-74-7
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryHebei Nengqian Chemical Import and Export Co., LTD
With our good experience, we offer detailed technical support and advice to assist customers. We communicate closely with customers to establish their quality requirements. Consistent Quality Our plant has strict quality control in each manufacturing
Cas:67843-74-7
Min.Order:1 Kilogram
FOB Price: $15.0 / 50.0
Type:Trading Company
inquiryZhuozhou Wenxi import and Export Co., Ltd
WITH US,YOUR MONEY IN SAFE,YOUR BUSINESS IN SAFE 1)Quick Response Within 12 hours; 2)Quality Guarantee: All products are strictly tested by our QC, confirmed by QA and approved by third party lab in China, USA, Canada, Germany, UK, Italy, France et
Cas:67843-74-7
Min.Order:1 Kilogram
FOB Price: $139.0 / 210.0
Type:Trading Company
inquiryWuhan Han Sheng New Material Technology Co.,Ltd
Our Advantage: high quality with competitive price High quality standard: BP/USP/EP Enterprise standard All purity customized Fast and safe delivery We have reliable forwarder who can help us deliver our goods more fast and safe. We
Hebei Sankai Chemical Technology Co., Ltd
1. Product advantages ♦ High purity, all above 98.5%, no impurities after dissolution ♦ We will test each batch to ensure quality ♦ OEM and private brand services designed for free ♦ Various cap colors available ♦ W
Cas:67843-74-7
Min.Order:1 Kilogram
FOB Price: $7.0 / 9.0
Type:Trading Company
inquiryHenan Wentao Chemical Product Co., Ltd.
We are leading fine chemicals supplier in China with ISO certificate, Our main business covers the fields below: 1.Noble Metal Catalysts (Pt.Pd...) 2.Organic Phosphine Ligands (Tert-butyl-phosphine.Cyclohexyl-phosphine...) 3.OLED
Cas:67843-74-7
Min.Order:1 Gram
FOB Price: $2.0
Type:Lab/Research institutions
inquiryHubei Langyou International Trading Co., Ltd
1. Guaranteed purity; 2. Large quantity in stock; 3. Largest manufacturer; 4. Best service after shipment with email; 5. High quality & competitive price; Appearance:white or off-white powder Storage:Store in sealed containers
Cas:67843-74-7
Min.Order:100 Gram
Negotiable
Type:Other
inquiryShanghai Upbio Tech Co.,Ltd
ProName: (S)-(+)-Epichlorohydrin 67843-74-7 CasNo: 67843-74-7 Molecular Formula: C3H5ClO Appearance: white powder Application: Usd for medical intermediate DeliveryTime: PROMPT PackAge: IN 25kg drums Port: Shanghai po
Cas:67843-74-7
Min.Order:100 Gram
FOB Price: $100.0
Type:Lab/Research institutions
inquiryBaoji Guokang Healthchem co.,ltd
Our company has been in existence for 10 years since its establishment. We have our own unique team. The company integrates independent research and development, production and sales. We have established famous brands at home and abroad. At present
Cas:67843-74-7
Min.Order:1 Kilogram
FOB Price: $234.0 / 255.0
Type:Trading Company
inquiryQingdao Beluga Import and Export Co., LTD
(S)-(+)-Epichlorohydrin CAS:67843-74-7 Qingdao Belugas Import and Export Co., Ltd. is a scientific and technological company integrating research and development, production and trade of chemical intermediates, specializing in high quality organic i
Cas:67843-74-7
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryShandong Hanjiang Chemical Co., Ltd.
Hello, dear friend! I'm Hansen and Allen from China. Welcome to my lookchem mall! The following is a brief introduction of our company's products and services. If you are interested in our products, please contact us by emai
Cas:67843-74-7
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryShanxi Ankesi Biotechnology Co., Ltd
Our Advantage 1. Rich experience We specialize in this filed for many years, our APIs exported to all over the world and and we established long friendly relations of cooperation with our clients. 2. Great quality,purity and favorable Good qual
Cas:67843-74-7
Min.Order:1 Kilogram
Negotiable
Type:Trading Company
inquiryHebei Mojin Biotechnology Co.,Ltd
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional chemical raw materials and chemical reagents research and development production enterprises. We have several production line,So we can control the lowest price. We also have several
Cas:67843-74-7
Min.Order:25 Gram
FOB Price: $90.0 / 100.0
Type:Trading Company
inquiryLonwin Chemical Group Limited
(S)-(+)-Epichlorohydrin CAS:67843-74-7 Specification Item Specification Appearance Clear Colorless to Very Pale Yellow Liquid Assay 98%min Density 1.183 g/mL at 25 °C(lit.) Boiling Poi
Cas:67843-74-7
Min.Order:1 Kilogram
Negotiable
Type:Other
inquiryWuhan Zenuo Biological Medicine Technology Co Ltd
Product Name: (S)-(+)-Epichlorohydrin Synonyms: (S)-(+)-Epichlorohydrin 67843-74-7;(S)-epichlorohydrin 67843-74-7 (S)-(+)-Epichlorohydrin;(S)-(+)-2-(Chloromethyl)oxirane (S)-Epichlorohydrin (S) 1-Chloro-2,3-epoxypropane (S)-3-Chloropropylene Oxi
Cas:67843-74-7
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryTriumph International Development Limilted
Lorcaserin(856681-05-5)is an orally administered agent and a selective 5-HT2C receptor agonist for the treatment of obesity. It had been approved for marketing in US by FDA on 27 June in 2012. In clinical studies, lorcaserin h
Cas:67843-74-7
Min.Order:100 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryHangzhou Keyingchem Co.,Ltd
Hangzhou KeyingChem Co., Ltd. exported this product to many countries and regions at best price. If you are looking for the material’s manufacturer or supplier in China, KeyingChem is your best choice. Pls contact with us freely for getting det
Cas:67843-74-7
Min.Order:0 Metric Ton
Negotiable
Type:Lab/Research institutions
inquiryTianjin Kind Pharma Co., Ltd.
Factory direct sales, accept customization. Kind Pharma has its own laboratory, we can do analytical testing for API (Active Pharmaceutical Ingredient), pharmaceutical intermediate, pesticide intermediate, additive and other chemical products, so it
Cas:67843-74-7
Min.Order:1 Kilogram
Negotiable
Type:Lab/Research institutions
inquiryAfine Chemicals Limited
Company Introduction 1. Established in 2005, with two independent business divisions: Fine chemicals division; Pharmaceutical division. 2. Main product: Optical brightener Textile auxiliary Dye stuff Pigments
Cas:67843-74-7
Min.Order:1 Gram
Negotiable
Type:Lab/Research institutions
inquiryHangzhou J&H Chemical Co., Ltd.
J&H CHEM R&D center can offer custom synthesis according to the contract research and development services for the fine chemicals, pharmaceutical, biotechnique and some of the other chemicals. J&H CHEM has some Manufacturing base in Jia
SHANGHAI T&W PHARMACEUTICAL CO., LTD.
A substitute for perfluorooctanoic acid, mainly used as a surfactant, dispersant, additive, etc Appearance:White solid or Colorless liquid Purity:99.3 % We will ship the goods in a timely manner as required We can provide relevant documents acc
Cas:67843-74-7
Min.Order:4 Kilogram
Negotiable
Type:Lab/Research institutions
inquirySynthetic route
-
-
96-23-1
1,3-Dichloro-2-propanol

-
-
67843-74-7
(S)-epichlorohydrin

| Conditions | Yield |
|---|---|
| With halohydrin dehalogenase from Agrobacterium radiobacter AD1 (HheC mutant P175S/W249P) In aq. phosphate buffer pH=8; Concentration; Reagent/catalyst; Temperature; pH-value; Solvent; Enzymatic reaction; enantioselective reaction; | 93.7% |
| With halohydrin dehalogenase mutant HheCPS F86N In aq. phosphate buffer at 37℃; pH=8; Catalytic behavior; Reagent/catalyst; Enzymatic reaction; enantioselective reaction; | 79.12% |
-
-
3132-64-7
1,2-Epoxy-3-bromopropane

-
A
-
67843-74-7
(S)-epichlorohydrin

-
B
-
14437-88-8
(2R)-3-bromo-1,2-propanediol

| Conditions | Yield |
|---|---|
| With (R,R)-Jacobsen catalyst; water In tetrahydrofuran at 4℃; for 24h; | A n/a B 93% |
-
-
621-84-1
O-benzyl carbamate

-
-
106-89-8
epichlorohydrin

-
A
-
67843-74-7
(S)-epichlorohydrin

-
B
-
641617-19-8
[(R)-3-chloro-2-hydroxypropyl]carbamic acid benzyl ester

| Conditions | Yield |
|---|---|
| With air; 4-nitro-benzoic acid; (R,R)-((t-Bu)4-salen)Co(II) In various solvent(s) at 0℃; for 24h; | A n/a B 87% |

-
-
67800-61-7
(S)-3-chloro-2-hydroxypropyl-1-(toluene-4-sulfonate)

-
-
67843-74-7
(S)-epichlorohydrin

| Conditions | Yield |
|---|---|
| With sodium ethane-1,2-diolate In ethylene glycol for 0.25h; Ambient temperature; | 85% |
-
-
106-89-8
epichlorohydrin

-
-
67843-74-7
(S)-epichlorohydrin

| Conditions | Yield |
|---|---|
| With water; [(1-RR)-(Dibenzoyl-LTA)] at 5 - 20℃; for 7h; Product distribution / selectivity; Industry scale; Resolution of racemate; | 82% |
| With water; [(1-RR)-(Dibenzoyl-LTA)] at 5 - 20℃; for 3h; Product distribution / selectivity; Resolution of racemate; | 80% |
| With oligomeric (salen)Co(OSO2CF3); water at 20℃; for 15h; | 44% |
-
-
616-23-9, 82890-22-0, 89460-14-0, 78692-89-4
(R)-2,3-Dichloro-1-propanol

-
-
67843-74-7
(S)-epichlorohydrin

| Conditions | Yield |
|---|---|
| With sodium hydroxide | 74% |
-
-
106-89-8
epichlorohydrin

-
A
-
67843-74-7
(S)-epichlorohydrin

-
B
-
57090-45-6
(2R)-3-chloro-1,2-propanediol

| Conditions | Yield |
|---|---|
| With C72H102Co2F12N4O12P2; water at 0 - 20℃; for 3h; optical yield given as %ee; | A 45% B 53% |
| With water; (S,S)-(salen)cobalt(III)(OAc) at 0℃; for 19h; | A 46% B 45% |
| With C114H155Co3N8O14Pol; water; acetic acid at 20℃; for 3h; Resolution of racemate; optical yield given as %ee; enantioselective reaction; | A 46% B n/a |

-
-
67843-74-7
(S)-epichlorohydrin

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol Ambient temperature; | 51% |
-
-
106-89-8
epichlorohydrin

-
A
-
67843-74-7
(S)-epichlorohydrin

-
B
-
57090-45-6
(2R)-3-chloro-1,2-propanediol

-
C
-
60827-45-4
(S)-3-chloropropan-1,2-diol

| Conditions | Yield |
|---|---|
| With water; dimeric chiral (salen)Co complex linked with Al at 20℃; for 5h; Product distribution; Further Variations:; Catalysts; | A 46% B n/a C n/a |
| With water; Cr(III)-endo,endo-2,5-diaminonorbornane-salen In tetrahydrofuran at 20℃; for 42h; | A 46% B n/a C n/a |
| With chiral oligo-(salen)Co(OTs) complexes; lutidinium p-toluene sulfonate; water In dichloromethane; acetonitrile at 20℃; for 11h; | A 45% B n/a C n/a |


| Conditions | Yield |
|---|---|
| Stage #1: epichlorohydrin With C57H54CoN3O8 In dichloromethane at 20℃; for 0.25h; Stage #2: With water In dichloromethane at 20℃; for 12h; Cooling with ice; enantioselective reaction; | A 46% B n/a |
-
-
106-89-8
epichlorohydrin

-
A
-
67843-74-7
(S)-epichlorohydrin

-
B
-
51594-55-9
(R)-(-)-epichlorohydrin

-
C
-
96-23-1
1,3-Dichloro-2-propanol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; tert-butyl methyl ether; dimeric chiral (salen)Co complex linked with Al In diethyl ether at 0 - 5℃; for 2h; Product distribution; Further Variations:; Catalysts; | A n/a B n/a C 45% |

-
-
14199-15-6
Methyl 4-hydroxyphenylacetate

-
-
106-89-8
epichlorohydrin

-
A
-
67843-74-7
(S)-epichlorohydrin

-
B
-
724776-52-7
methyl (R)-2-(4-(3-chloro-2-hydroxypropoxy)phenyl)acetate

| Conditions | Yield |
|---|---|
| With (R,R)-Jacobsen(III)- catalyst immobilized by sulfonic acid functionalized SBA-16 mesoporous silica In tetrahydrofuran at 20℃; for 12h; optical yield given as %ee; enantioselective reaction; | A 44% B n/a |

-
-
106-89-8
epichlorohydrin

-
-
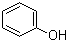
108-95-2
phenol

-
A
-
67843-74-7
(S)-epichlorohydrin

-
B
-
140630-45-1
(R)-1-chloro-3-phenoxy-2-propanol

| Conditions | Yield |
|---|---|
| With (R,R)-Jacobsen(III)- catalyst immobilized by sulfonic acid functionalized SBA-16 mesoporous silica In tetrahydrofuran at 20℃; for 12h; optical yield given as %ee; enantioselective reaction; | A 43% B n/a |

-
-
106-89-8
epichlorohydrin

-
A
-
67843-74-7
(S)-epichlorohydrin

-
B
-
51594-55-9
(R)-(-)-epichlorohydrin

-
C
-
57090-45-6
(2R)-3-chloro-1,2-propanediol

| Conditions | Yield |
|---|---|
| With (S,S)-[N,N-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexane-diamino]cobalt(III) acetate; (salen)Co(III)-AlCl3; water In tetrahydrofuran at 19.84℃; for 3h; Kinetics; Reagent/catalyst; Solvent; optical yield given as %ee; enantioselective reaction; | A n/a B n/a C 41% |
| With C8F17COOH; water; (R,R)-Co(III)(salen) In toluene at 20℃; for 15h; | A n/a B n/a C 40% |
| With chiral oligo-(salen)Co(OTs) complexes; lutidinium p-toluene sulfonate; water In dichloromethane; acetonitrile at 0 - 20℃; for 1.5h; | A n/a B n/a C 40% |


| Conditions | Yield |
|---|---|
| With Agromyces mediolanus ZJB120203 epoxide hydrolase Resolution of racemate; enantioselective reaction; | A 21.5% B n/a |
| enantiomeric resolution by complexation gas chromatography on nickel(II)bis<(1R)-3-(heptafluorobutyryl)camphorate>; | |
| With water; C6H15N*C44H61CoN2O10 at 5 - 20℃; for 3 - 9h; Product distribution / selectivity; Resolution of racemate; |
-
-
96-23-1
1,3-Dichloro-2-propanol

-
A
-
67843-74-7
(S)-epichlorohydrin

-
B
-
51594-55-9
(R)-(-)-epichlorohydrin

| Conditions | Yield |
|---|---|
| With potassium carbonate; Co(II)(3,5-Cl,Cl-sal)2(S-CHXDA) (e.e. 1.) 130-150 deg C, 3 hr in vacuo; 2.) CH2Cl2, 25 deg C, 6 days; Yield given. Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; | |
| With potassium carbonate In dichloromethane at 25℃; Product distribution; Mechanism; study of asymmetric cyclization using different optically active cobalt (salen) or nickel (salen) type complexes; | |
| With potassium phosphate; [(R,R)-(salen)Co(II)]2*Al(NO3)3 In tetrahydrofuran at 20℃; for 3h; |
-
-
67800-61-7
(S)-3-chloro-2-hydroxypropyl-1-(toluene-4-sulfonate)

-
A
-
67843-74-7
(S)-epichlorohydrin

-
B
-
51594-55-9
(R)-(-)-epichlorohydrin

| Conditions | Yield |
|---|---|
| With sodium 2-hydroxyethoxide In ethylene glycol for 0.0833333h; Title compound not separated from byproducts; |
-
-
106-89-8
epichlorohydrin

-
A
-
67843-74-7
(S)-epichlorohydrin

-
B
-
51594-55-9
(R)-(-)-epichlorohydrin

-
C
-
57090-45-6
(2R)-3-chloro-1,2-propanediol

-
D
-
60827-45-4
(S)-3-chloropropan-1,2-diol

| Conditions | Yield |
|---|---|
| With water; poly(Co(III)(OTs)-salen-norbornene) In chlorobenzene at 20℃; for 0.5h; Product distribution; Further Variations:; Catalysts; reaction times; | |
| Co(Salen)/SBA-16-C8 In tetrahydrofuran; water at 24.84℃; for 20h; | |
| With C118H146Co2N4O14S2; water In acetonitrile at 20℃; optical yield given as %ee; enantioselective reaction; |

-
-
51594-55-9
(R)-(-)-epichlorohydrin

-
-
67843-74-7
(S)-epichlorohydrin

| Conditions | Yield |
|---|---|
| With sodium citrate buffer; Agrobacterium radiobacter AD1 haloalcohol dehalogenase; sodium chloride at 22℃; pH=5.5; Product distribution; Further Variations:; pH-values; | |
| With Agrobacterium radiobacter halohydrin dehalogenase; sodium chloride for 1h; pH=7.5; Tris-SO4 buffer; Enzymatic reaction; |
-
-
4248-19-5
tert-butyl carbamate

-
-
106-89-8
epichlorohydrin

-
A
-
67843-74-7
(S)-epichlorohydrin

-
B
-
1072792-33-6
(R)-3-chloro-2-hydroxypropylcarbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With (1R,2R)-(-)-N,N'-bis(3,5-di-tert-butylsalicydene)-1,2-cyclohexanediaminocobalt(II); 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate; 4-nitro-benzoic acid at 20℃; for 8h; optical yield given as %ee; enantioselective reaction; | |
| Stage #1: tert-butyl carbazate With chiral Co(III) polymeric salen complex In dichloromethane for 0.166667h; Stage #2: epichlorohydrin In dichloromethane for 20h; enantioselective reaction; | A n/a B n/a |

-
-
51594-55-9
(R)-(-)-epichlorohydrin

-
A
-
67843-74-7
(S)-epichlorohydrin

-
B
-
57090-45-6
(2R)-3-chloro-1,2-propanediol

| Conditions | Yield |
|---|---|
| With (R,R)-Jacobsen catalyst; water | A n/a B n/a |
-
-
62-53-3
aniline

-
-
106-89-8
epichlorohydrin

-
A
-
67843-74-7
(S)-epichlorohydrin

-
B
-
51594-55-9
(R)-(-)-epichlorohydrin

-
C
-
195455-97-1
(S)-(+)-1-chloro-3-(phenylamino)-propan-2-ol

-
D
-
195455-98-2
(R)-(+)-N-(3-chloro-2-hydroxypropyl)aniline

| Conditions | Yield |
|---|---|
| Stage #1: epichlorohydrin With C65H56CoN3O8 In dichloromethane at 27 - 28℃; for 0.166667h; Stage #2: aniline In dichloromethane at 27 - 28℃; enantioselective reaction; | A n/a B n/a C n/a D n/a |

-
-
590-28-3
potassium cyanate

-
-
67843-74-7
(S)-epichlorohydrin

-
-
169048-83-3
(5S)-5-(chloromethyl)-1,3-oxazolidin-2-one

| Conditions | Yield |
|---|---|
| In water for 15h; Heating; | 100% |
| With water for 15h; Reflux; | 60% |
| With magnesium sulfate In water at 100℃; for 5h; | 41% |
| In water Reflux; | |
| In water for 19h; Reflux; |
-
-
67843-74-7
(S)-epichlorohydrin

-
-
777934-39-1
2-benzylamino-N-(4-benzyloxyphenyl)acetamide

-
-
777934-41-5
2-{benzyl[(2S)-3-chloro-2-hydroxypropyl]amino}-N-(4-benzyloxyphenyl)acetamide

| Conditions | Yield |
|---|---|
| With magnesium sulfate In methanol; dichloromethane at 35℃; for 72h; | 100% |
| With magnesium sulfate In methanol; dichloromethane at 35℃; for 72h; |
-
-
67843-74-7
(S)-epichlorohydrin

-
-
107-07-3
2-chloro-ethanol

-
-
861852-07-5
(S)-1-(2-chloroethoxy)-3-chloropropan-2-ol

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate at 45℃; for 1.5h; | 100% |
| With boron trifluoride diethyl etherate at 45℃; for 1.5h; | 96% |
| With boron trifluoride diethyl etherate In toluene at 36 - 38℃; Large scale reaction; |

| Conditions | Yield |
|---|---|
| Stage #1: bromopentene With magnesium In tetrahydrofuran for 0.5h; Heating; Stage #2: (S)-epichlorohydrin In tetrahydrofuran at -50 - 20℃; for 2h; | 100% |
-
-
67843-74-7
(S)-epichlorohydrin

-
-
327-52-6
1-bromo-2,4,5-trifluorobenzene

-
-
1246960-15-5
(S)-1-chloro-3-(2,4,5-trifluorophenyl)propan-2-ol

| Conditions | Yield |
|---|---|
| Stage #1: 1-bromo-2,4,5-trifluorobenzene With isopropylmagnesium chloride In tetrahydrofuran at -20 - 3℃; for 1.16667h; Inert atmosphere; Stage #2: With copper(l) iodide In tetrahydrofuran at -10℃; for 0.5h; Inert atmosphere; Stage #3: (S)-epichlorohydrin In tetrahydrofuran at -10 - 0℃; for 2.5h; Inert atmosphere; | 100% |
| Stage #1: 1-bromo-2,4,5-trifluorobenzene With magnesium; 1,2-dibromomethane In tetrahydrofuran at 20℃; Stage #2: (S)-epichlorohydrin With copper(l) iodide In tetrahydrofuran at 0 - 20℃; for 2.5h; Product distribution / selectivity; | |
| Stage #1: 1-bromo-2,4,5-trifluorobenzene With magnesium; ethylene dibromide In tetrahydrofuran at 20℃; Stage #2: (S)-epichlorohydrin; copper(l) iodide In tetrahydrofuran at 0 - 20℃; for 2.5h; Product distribution / selectivity; | |
| Stage #1: 1-bromo-2,4,5-trifluorobenzene With isopropylmagnesium chloride In tetrahydrofuran at 0 - 22℃; for 4h; Stage #2: (S)-epichlorohydrin With copper(l) chloride In tetrahydrofuran at 25 - 40℃; for 5h; Stage #3: With acetic acid In tetrahydrofuran; tert-butyl methyl ether |
-
-
67843-74-7
(S)-epichlorohydrin

-
-
3218-49-3
3,4-dichloro-benzeneacetonitrile

-
-
910028-19-2
((1S,2R)-2-(aminomethyl)-2-(3,4-dichlorophenyl)cyclopropyl)methanol

| Conditions | Yield |
|---|---|
| Stage #1: (S)-epichlorohydrin; 3,4-dichloro-benzeneacetonitrile With sodium hexamethyldisilazane In tetrahydrofuran at -15 - 4℃; Inert atmosphere; Stage #2: With dimethylsulfide borane complex at -5 - 40℃; for 6.5h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: isopropenylmagnesium bromide With copper(l) iodide; boron trifluoride diethyl etherate In tetrahydrofuran at -78 - -30℃; for 0.5h; Inert atmosphere; Stage #2: (S)-epichlorohydrin In tetrahydrofuran at -30℃; for 0.5h; Inert atmosphere; Stage #3: With sodium hydroxide In dichloromethane at 35℃; for 30h; Inert atmosphere; | 99% |
| Stage #1: isopropenylmagnesium bromide With copper(l) iodide In tetrahydrofuran at -78℃; for 0.5h; Inert atmosphere; Stage #2: (S)-epichlorohydrin In tetrahydrofuran at -30℃; for 0.5h; Inert atmosphere; Stage #3: With sodium hydroxide In dichloromethane at 35℃; for 30h; Inert atmosphere; | 10.29 g |
-
-
67843-74-7
(S)-epichlorohydrin

-
-
103-49-1
dibenzylamine

-
-
565176-84-3
(S)-N,N-dibenzylamino-1-(oxiran-2-ylmethyl)methylamine

| Conditions | Yield |
|---|---|
| Stage #1: (S)-epichlorohydrin; dibenzylamine In isopropyl alcohol at 0 - 20℃; Stage #2: With potassium hydroxide In isopropyl alcohol at 0℃; | 98.5% |
| With sodium hydroxide at 65 - 70℃; for 24h; | 95% |
| In isopropyl alcohol at 0℃; for 24h; | 91.5% |
| Stage #1: (S)-epichlorohydrin; dibenzylamine In methanol at 20℃; for 48h; Stage #2: With potassium hydroxide In water; tert-butyl alcohol at 20℃; for 48h; | 45.8% |

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate | 98% |
-
-
1121-24-0
ortho-mercaptophenol

-
-
67843-74-7
(S)-epichlorohydrin

-
-
1588421-61-7
(R)-3-[(2-hydroxyphenyl)thio]-1,2-epoxypropane

| Conditions | Yield |
|---|---|
| With pyridine In water at 20℃; for 24h; enantiospecific reaction; | 98% |
| Stage #1: ortho-mercaptophenol With pyridine In N,N-dimethyl-formamide at 20℃; for 0.0833333h; Inert atmosphere; Stage #2: (S)-epichlorohydrin In N,N-dimethyl-formamide at 140℃; for 0.166667h; Inert atmosphere; Microwave irradiation; |
-
-
67843-74-7
(S)-epichlorohydrin

| Conditions | Yield |
|---|---|
| Stage #1: N-[4-(3-morpholinon-4-yl)phenyl]-2-thiophene chloride With lithium tert-butoxide In tetrahydrofuran at 20℃; for 0.333333h; Stage #2: (S)-epichlorohydrin at 50 - 60℃; for 3h; | 98% |
-
-
67843-74-7
(S)-epichlorohydrin

-
-
103-49-1
dibenzylamine

-
-
1236384-02-3
(S)-3-chloro-2-hydroxypropyl dibenzylamine

| Conditions | Yield |
|---|---|
| With calcium chloride at 33℃; under 608.041 - 912.061 Torr; for 8h; Product distribution / selectivity; | 97% |
| With calcium chloride In dichloromethane at 33℃; for 8h; Product distribution / selectivity; | 97% |
| In dichloromethane at 25℃; for 20h; Solvent; Temperature; | 95.16% |
| In ethanol for 6h; Reflux; | 28.3 g |
| at 25 - 30℃; for 18h; |
-
-
67843-74-7
(S)-epichlorohydrin

-
-
1066-54-2
trimethylsilylacetylene

-
-
148516-13-6
(S)-1-chloro-2-hydroxy-5-trimethylsilyl-4-pentyne

| Conditions | Yield |
|---|---|
| Stage #1: trimethylsilylacetylene With n-butyllithium In tetrahydrofuran; hexane at -60℃; for 0.5h; Stage #2: (S)-epichlorohydrin With boron trifluoride diethyl etherate In tetrahydrofuran; hexane at -60℃; for 2h; | 96% |
| With n-butyllithium; boron trifluoride diethyl etherate In tetrahydrofuran; hexane at -60℃; for 2h; | 96% |
| With n-butyllithium; boron trifluoride diethyl etherate 1.) THF, -78 deg C, 25 min, 2.) THF, -30 deg C, 18 h; Multistep reaction; |
-
-
67-56-1
methanol

-
-
67843-74-7
(S)-epichlorohydrin

-
-
201230-82-2
carbon monoxide

-
-
86728-93-0
methyl (S)-4-chloro-3-hydroxybutanoate

| Conditions | Yield |
|---|---|
| With 3-HYDROXYPYRIDINE; dicobalt octacarbonyl In tetrahydrofuran at 55℃; under 31028.9 Torr; for 9h; | 96% |
| 4-aminopyridine; dicobalt octacarbonyl In tert-butyl alcohol at 40℃; under 7500.75 Torr; for 5h; Product distribution / selectivity; | 92% |
| 4-aminopyridine; dicobalt octacarbonyl at 33℃; under 7500.75 Torr; for 25h; Product distribution / selectivity; | 85% |

-
-
67843-74-7
(S)-epichlorohydrin

-
-
5294-61-1
N-(2,6-dimethylphenyl)-2-(piperazin-1-yl)acetamide

-
-
1427177-25-0
(S)-2-(4-(3-chloro-2-hydroxypropyl)piperazin-1-yl)-N-(2,6-dimethylphenyl)acetamide

| Conditions | Yield |
|---|---|
| In water at 15℃; for 1h; | 96% |

-
-
67843-74-7
(S)-epichlorohydrin

| Conditions | Yield |
|---|---|
| Stage #1: (R)-2-hydroxy-2'-(methoxymethoxy)-1,1'-binaphthalene With tetrabutylammomium bromide; potassium hydroxide In tetrahydrofuran at 20℃; for 0.166667h; Stage #2: (S)-epichlorohydrin In tetrahydrofuran at 20℃; for 20h; | 96% |
-
-
67843-74-7
(S)-epichlorohydrin

| Conditions | Yield |
|---|---|
| Stage #1: N-[4-(3-morpholinon-4-yl)phenyl]-5-chloro-2-thiophenecarboxamide With lithium tert-butoxide In tetrahydrofuran at 20℃; for 0.333333h; Stage #2: (S)-epichlorohydrin at 50 - 60℃; for 3h; | 96% |
-
-
67843-74-7
(S)-epichlorohydrin

| Conditions | Yield |
|---|---|
| With dilithium tetrabromonickelate(II) In tetrahydrofuran 1) 0 degC, 2 h, 2) r.t., 1 h; | 95% |
-
-
67843-74-7
(S)-epichlorohydrin

-
-
56522-24-8
tert-butyldimethylsilyl cyanide

-
-
884902-55-0
(3S)-3-[(tert-butyldimethylsilyl)oxy]-4-chlorobutanenitrile

| Conditions | Yield |
|---|---|
| With potassium cyanide; 18-crown-6 ether | 95% |
-
-
67843-74-7
(S)-epichlorohydrin

-
-
791856-68-3
6-(4-bromophenyl)-1,2,3,4-tetrahydro-1,5-naphthyridine

-
-
944152-87-8
(+)-(S)-6-(4-bromophenyl)-1-(3-chloro-2-hydroxypropyl)-1,2,3,4-tetrahydro-1,5-naphthyridine

| Conditions | Yield |
|---|---|
| With ytterbium(III) triflate In dichloromethane at 60℃; for 5h; | 95% |
-
-
67843-74-7
(S)-epichlorohydrin

-
-
107-92-6
butyric acid

-
-
890051-54-4
butyric acid-3-chloro-(S)-2-hydroxy-propyl ester

| Conditions | Yield |
|---|---|
| With chromium chloride at 60℃; for 24h; | 95% |
| Stage #1: butyric acid With N,N'-bis(3,5-di-tert-butylsalicylidene)ethylenediaminocobalt(II); oxygen at 50℃; for 1h; Stage #2: (S)-epichlorohydrin With diisopropylamine at 20℃; for 24h; | 82.0 %Chromat. |
-
-
693-03-8
n-butyl magnesium bromide

-
-
67843-74-7
(S)-epichlorohydrin

-
-
1224174-07-5
(S)-1-chlorohept-6-en-2-ol

| Conditions | Yield |
|---|---|
| With copper(l) iodide In tetrahydrofuran at -78℃; | 95% |
-
-
67843-74-7
(S)-epichlorohydrin

-
-
57260-71-6
1-t-Butoxycarbonylpiperazine

-
-
335165-58-7
tert-butyl 4-[[(2S)-oxiran-2-yl]methyl]piperazine-1-carboxylate

| Conditions | Yield |
|---|---|
| In ethanol for 12h; | 95% |
-
-
67843-74-7
(S)-epichlorohydrin

-
-
100-51-6
benzyl alcohol

-
-
2930-05-4, 14618-80-5, 89616-40-0, 16495-13-9
(S)-benzyl glycidyl ether

| Conditions | Yield |
|---|---|
| Stage #1: (S)-epichlorohydrin With tetrabutylammomium bromide; sodium hydroxide In water at 20℃; Stage #2: benzyl alcohol In water at 20℃; regioselective reaction; | 95% |
-
-
67843-74-7
(S)-epichlorohydrin

-
-
92-66-0
4-bromo-1,1'-biphenyl

-
-
1573000-28-8
(S)-1-([1,1’-biphenyl]-4-yl)-3-chloropropan-2-ol

| Conditions | Yield |
|---|---|
| Stage #1: 4-bromo-1,1'-biphenyl With iodine; magnesium In tetrahydrofuran at 40 - 50℃; for 2h; Stage #2: (S)-epichlorohydrin With copper(l) iodide In tetrahydrofuran at -15 - 5℃; for 2h; | 95% |
| Stage #1: 4-bromo-1,1'-biphenyl With iodine; magnesium In tetrahydrofuran at 45 - 50℃; for 0.166667h; Stage #2: With copper(l) iodide In tetrahydrofuran at -10 - 5℃; for 0.5h; Stage #3: (S)-epichlorohydrin In tetrahydrofuran for 4h; | 92% |
| Stage #1: 4-bromo-1,1'-biphenyl With n-butyllithium In tetrahydrofuran; hexane at -78 - -10℃; for 2.5h; Inert atmosphere; Stage #2: (S)-epichlorohydrin In tetrahydrofuran; hexane at 20℃; for 5h; | 85% |
| Stage #1: 4-bromo-1,1'-biphenyl With iodine; magnesium In tetrahydrofuran at 45 - 50℃; Inert atmosphere; Industrial scale; Stage #2: With copper(l) iodide In tetrahydrofuran at -20 - -15℃; for 0.5h; Industrial scale; Stage #3: (S)-epichlorohydrin Reagent/catalyst; Temperature; Industrial scale; Further stages; | 48.7 kg |
| Stage #1: 4-bromo-1,1'-biphenyl With iodine; magnesium In tetrahydrofuran at 35 - 45℃; Inert atmosphere; Stage #2: (S)-epichlorohydrin With copper(l) iodide In tetrahydrofuran at -20 - -15℃; Inert atmosphere; | 45.4 g |
-
-
67843-74-7
(S)-epichlorohydrin

-
-
3695-77-0
triphenylmethanethiol

| Conditions | Yield |
|---|---|
| With potassium fluoride In methanol at 20℃; for 72h; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: n-Butyl chloride With iodine; magnesium In tetrahydrofuran at 80℃; for 1h; Inert atmosphere; Stage #2: (S)-epichlorohydrin With copper(l) cyanide In tetrahydrofuran at -78 - -20℃; for 3h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 75℃; for 0.25h; | 95% |
-
-
67843-74-7
(S)-epichlorohydrin

-
-
89343-06-6
tris-iso-propylsilyl acetylene

-
-
638222-25-0
(2S)-1-chloro-5-(triisopropylsilanyl)pent-4-yn-2-ol

| Conditions | Yield |
|---|---|
| Stage #1: tris-iso-propylsilyl acetylene With n-butyllithium; boron trifluoride-tetrahydrofuran complex In tetrahydrofuran; hexane at -78℃; for 1h; Stage #2: (S)-epichlorohydrin In tetrahydrofuran; hexane at -78℃; for 2h; Hiroa reaction; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: ethyl bromide With iodine; magnesium In diethyl ether for 1.5h; Reflux; Stage #2: (S)-epichlorohydrin With copper(l) cyanide In tetrahydrofuran; diethyl ether at -78 - -20℃; for 3h; Stage #3: With ammonium chloride In tetrahydrofuran; diethyl ether; water | 94% |
Related products
Raw Materials
Downstream Products
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View















 T
T