-
Name
TRANS-BETA-NITROSTYRENE
- EINECS 203-066-0
- CAS No. 102-96-5
- Article Data192
- CAS DataBase
- Density 1.177g/cm3
- Solubility
-
Melting Point
55-58 °C(lit.)
- Formula C8H7 N O2
-
Boiling Point
250-260 °C(lit.)
- Molecular Weight 149.149
- Flash Point 117.5°C
- Transport Information
- Appearance yellow crystals
- Safety Moderately toxic by ingestion. When heated to decomposition it emits toxic vapors of NOx.
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols

- Synonyms Styrene, b-nitro- (8CI);(2-Nitroethenyl)benzene; (2-Nitrovinyl)benzene; 1-Nitro-2-phenylethene;1-Nitro-2-phenylethylene; 1-Phenyl-2-nitroethene; 2-Nitro-1-phenylethylene;2-Phenyl-1-nitroethene; NSC 9809; b-Nitrostyrene; w-Nitrostyrene
- PSA 45.82000
- LogP 2.45720
Synthetic route

| Conditions | Yield |
|---|---|
| With 3-(diethylamino)propyltrimethoxysilane supported on silica-alumina at 100℃; for 6h; | 99% |
| With mesoporous hybrid catalyst HYB-75P-25B at 373℃; for 14h; Henry reaction; Inert atmosphere; | 98% |
| With basic groups-derivatized mesoporous silica-enveloped Fe3O4 nanoparticles Henry Nitro Aldol Condensation; | 98% |
-
-
75-52-5
nitromethane

-
-
1125-88-8
benzaldehyde dimethyl acetal

-
A
-
102-96-5
(2-nitroethenyl)benzene

-
B
-
100-52-7
benzaldehyde

| Conditions | Yield |
|---|---|
| With benzenesulfonic acid and amine group containing periodic mesoporous organosilica at 90℃; for 20h; | A 97.5% B 2.5% |
| With DMAN-SO3H-SiO2-5-5 In water at 89.84℃; for 24h; Catalytic behavior; Time; Henry Nitro Aldol Condensation; | A 92% B 8% |
| With grDMAN-SO3H-SiO2-5 In water at 89.84℃; for 15h; Catalytic behavior; Time; Henry Nitro Aldol Condensation; | A 15% B 85% |


| Conditions | Yield |
|---|---|
| With porous aromatic framework PAF-1-NHCH2CH2NH2-SO3H at 90℃; for 24h; Inert atmosphere; | 97.2% |
| With acidic-basic groups-derivatized mesoporous silica-enveloped Fe3O4 nanoparticles In water at 90℃; for 5h; | 96% |
| With water at 90℃; for 5h; Inert atmosphere; | 95 %Chromat. |

| Conditions | Yield |
|---|---|
| With copper(II) nitrate In acetonitrile at 85℃; for 5h; Inert atmosphere; | 97% |
| With diethyl ether; mixture of gaseous nitrogen oxides | |
| With sodium nitrite |

-
-
102-96-5
(2-nitroethenyl)benzene

| Conditions | Yield |
|---|---|
| With sodium dihydrogen phosphate monohydrate; dichloro[1,1'-bis(di-t-butylphosphino)ferrocene]palladium(II); sodium nitrite In toluene at 120℃; for 0.5h; Temperature; Reagent/catalyst; Solvent; Microwave irradiation; Inert atmosphere; | 92% |
-
-
141377-54-0, 145920-96-3, 149495-00-1, 15990-45-1
2-nitro-1-phenylethan-1-ol

-
-
102-96-5
(2-nitroethenyl)benzene

| Conditions | Yield |
|---|---|
| With N-heterocyclic carbenes modified magnesium nanoparticles In tetrahydrofuran at 60℃; for 20h; Reagent/catalyst; Temperature; Molecular sieve; | 85% |

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite; silica gel In acetonitrile at 100℃; under 2250.23 Torr; Time; Temperature; Pressure; Solvent; Microwave irradiation; | 85% |
| With Vilsmeier reagent; potassium nitrate In acetonitrile at 20℃; Reagent/catalyst; Temperature; Sonication; | 82% |
| With nitric acid In acetonitrile for 0.025h; Solvent; Microwave irradiation; | 78% |
| With trichloroisocyanuric acid; N,N-dimethyl-formamide; sodium nitrite for 8h; Reflux; | 72% |
| Multi-step reaction with 2 steps 1: lime/chalk/ 2: nitric acid View Scheme |
-
-
292638-84-7
styrene

-
-
102-96-5
(2-nitroethenyl)benzene

| Conditions | Yield |
|---|---|
| With iodine; sodium nitrite In water; ethylene glycol; ethyl acetate for 48h; Ambient temperature; | 81% |
| With silver nitrate; acetyl chloride In acetonitrile at 0 - 65℃; for 1h; | 55% |
| With tert.-butylnitrite In dimethyl sulfoxide at 80℃; for 24h; Inert atmosphere; | 26% |
-
-
17356-08-0
thiourea

-
A
-
102-96-5
(2-nitroethenyl)benzene

-
B
-
1071-86-9, 19040-64-3, 50606-57-0
bis(formamidine)disulphide dihydrobromide

| Conditions | Yield |
|---|---|
| With (1,2-dibromo-2-nitroethyl)benzene In acetone | A n/a B 81% |
-
-
292638-84-7
styrene

-
A
-
102-96-5
(2-nitroethenyl)benzene

-
B
-
21205-24-3
2-nitro-1-phenylethan-1-one oxime

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite; water In dimethyl sulfoxide at 50℃; for 1.5h; | A 6% B 81% |
-
-
75-52-5
nitromethane

-
-
100-52-7
benzaldehyde

-
A
-
102-96-5
(2-nitroethenyl)benzene

-
B
-
117538-84-8
(1,3-dinitropropan-2-yl)benzene

| Conditions | Yield |
|---|---|
| With hexan-1-amine; silica gel In toluene at 90℃; for 1.5h; | A 80% B 14% |
| With cinchona alkaloid and aminopropyl group-based hybrid organic-inorganic catalyst In toluene at 70℃; for 5h; Henry Nitro Aldol Condensation; | A 51% B 18% |
| With ethylenediamine In water; toluene at 70℃; for 10h; Reagent/catalyst; Henry Nitro Aldol Condensation; | A 40% B 40% |
| With silica-alumina-supported amine catalyst at 100℃; for 8h; | A 71 %Spectr. B 29 %Spectr. |

-
-
75-52-5
nitromethane

-
-
100-52-7
benzaldehyde

-
A
-
102-96-5
(2-nitroethenyl)benzene

-
B
-
141377-54-0, 145920-96-3, 149495-00-1, 15990-45-1
2-nitro-1-phenylethan-1-ol

| Conditions | Yield |
|---|---|
| With ammonium acetate at 90℃; for 0.416667h; Henry reaction; Microwave irradiation; | A 71% B 12% |
| With ammonium acetate at 60℃; for 0.75h; Henry reaction; Ultrasound irradiation; | A 21% B 57% |
| With amino-functionalized MIL-101 catalyst In butan-1-ol at 80℃; for 8h; |

-
-
1345735-45-6
2-(2-nitro-1-phenylethoxy)-5-phenyltetrahydro-1,2-oxazole-3,3-dicarbonitrile

-
A
-
102-96-5
(2-nitroethenyl)benzene

-
B
-
61207-10-1
5-phenyl-4,5-dihydro-1,2-oxazole-3-carbonitrile 2-oxide

| Conditions | Yield |
|---|---|
| With water; acetic acid for 1h; Reflux; | A 52% B 70% |

| Conditions | Yield |
|---|---|
| With 4-(4-fluorophenyl)naphthalene-1,2-dione; oxygen at 80℃; for 12h; | 67% |
-
-
3425-99-8
(1,2-dibromo-2-nitroethyl)benzene

-
A
-
102-96-5
(2-nitroethenyl)benzene

-
B
-
1071-86-9, 19040-64-3, 50606-57-0
bis(formamidine)disulphide dihydrobromide

| Conditions | Yield |
|---|---|
| With thiourea In ethanol at 20 - 25℃; for 24h; | A n/a B 62.5% |
-
-
292638-84-7
styrene

-
A
-
102-96-5
(2-nitroethenyl)benzene

| Conditions | Yield |
|---|---|
| With Nitrogen dioxide; ozone In dichloromethane at -20℃; Product distribution; multistep reaction; | A 58% B n/a C n/a |

-
-
75-52-5
nitromethane

-
-
100-52-7
benzaldehyde

-
A
-
102-96-5
(2-nitroethenyl)benzene

-
B
-
145920-96-3
(R)-2-nitro-1-phenylethanol

| Conditions | Yield |
|---|---|
| With copper(II) acetate monohydrate; (3aR,3a'R,8aS,8a'S)-2,2'-(propane-2,2-diyl)bis(8,8a-dihydro-3aH-indeno[1,2-d]oxazole) In ethanol at 20℃; for 24h; Henry reaction; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | A 10% B 58% |


| Conditions | Yield |
|---|---|
| With tert.-butylnitrite; oxygen In water; dimethyl sulfoxide at 50℃; for 1.5h; | A 38% B 32% |
-
-
292638-84-7
styrene

-
-
10102-44-0
Nitrogen dioxide

-
A
-
102-96-5
(2-nitroethenyl)benzene

-
B
-
141377-54-0, 145920-96-3, 149495-00-1, 15990-45-1
2-nitro-1-phenylethan-1-ol

-
C
-
100-52-7
benzaldehyde

-
D
-
65-85-0
benzoic acid

-
E
-
614-21-1
2-nitroacetophenone

| Conditions | Yield |
|---|---|
| In tetrachloromethane react. at 25°C under N2 (anaerobic), further products; products identified by g.c./m.s.; | A 1% B 33% C 7% D 5% E 31% |
| In tetrachloromethane react. at 25°C, further products; products identified by g.c./m.s.; | A 12% B 24% C 9% D 1% E 29% |

-
-
75-52-5
nitromethane

-
-
100-52-7
benzaldehyde

-
A
-
102-96-5
(2-nitroethenyl)benzene

-
B
-
141377-54-0, 145920-96-3, 149495-00-1, 15990-45-1
2-nitro-1-phenylethan-1-ol

-
C
-
117538-84-8
(1,3-dinitropropan-2-yl)benzene

| Conditions | Yield |
|---|---|
| With N,N-dimethylethylenediamine In toluene at 50℃; for 1h; Henry Nitro Aldol Condensation; | A 32% B 18% C 18% |
| With 1,8-bis(tetramethylguanidino)naphthalene In neat (no solvent) at 129.84℃; Reagent/catalyst; Time; Henry Nitro Aldol Condensation; Inert atmosphere; | A 7% B 13% C 16% |
| With silica-alumina-supported double-amine catalyst at 100℃; for 8h; | A 3 %Spectr. B 4 %Spectr. C 93 %Spectr. |
| With silica-alumina-supported amine catalyst at 100℃; for 8h; | A 68 %Spectr. B 7 %Spectr. C 8 %Spectr. |
| With silica-alumina(500)-NEt2 at 100℃; for 20h; |

-
-
1707-07-9
dimethyl 2-phenylethenylphosphonate

-
A
-
102-96-5
(2-nitroethenyl)benzene

-
B
-
78728-63-9
dimethyl 2-hydroxy-1-nitro-2-phenylethylphosphonate

-
C
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| With dinitrogen tetraoxide at 0 - 20℃; for 3h; Nitration; | A n/a B 30% C n/a |


| Conditions | Yield |
|---|---|
| With potassium hydroxide |
-
-
75-52-5
nitromethane

-
-
96-15-1
2-Methylbutylamine

-
-
100-52-7
benzaldehyde

-
-
102-96-5
(2-nitroethenyl)benzene

-
-
75-52-5
nitromethane

-
-
141-52-6
sodium ethanolate

-
-
100-52-7
benzaldehyde

-
-
102-96-5
(2-nitroethenyl)benzene

| Conditions | Yield |
|---|---|
| Zerlegen des Produktes mit verd.Schwefelsaeure; |

-
-
75-52-5
nitromethane

-
-
100-52-7
benzaldehyde

-
-
5435-44-9, 22243-66-9
(E)-3-Ureido-but-2-enoic acid ethyl ester

-
-
102-96-5
(2-nitroethenyl)benzene

| Conditions | Yield |
|---|---|
| at 160℃; im geschlossenen Rohr; |

-
-
21080-09-1
NSC 4573

-
-
100-52-7
benzaldehyde

-
A
-
102-96-5
(2-nitroethenyl)benzene

-
B
-
538-51-2
benzylidene phenylamine

-
-
75-52-5
nitromethane

-
-
64-17-5
ethanol

-
-
134-81-6
benzil

-
A
-
102-96-5
(2-nitroethenyl)benzene

-
B
-
141377-54-0, 145920-96-3, 149495-00-1, 15990-45-1
2-nitro-1-phenylethan-1-ol

| Conditions | Yield |
|---|---|
| Natriumverbindung reagiert, Einw. von Mineralsaeuren; |


| Conditions | Yield |
|---|---|
| With pyridine; sodium ethanolate Behandlung des Reaktionsgemisches mit wss. HCl; |
-
-
42604-93-3
1-Phenyl-2-nitroethyl-p-chlorphenylsulfon

-
A
-
102-96-5
(2-nitroethenyl)benzene

-
B
-
100-03-8
4-chlorobenzenesulfinic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In acetone at 20℃; Rate constant; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| With polymer-bound NADH (2a) | 100% |
| With magnesium(II) perchlorate; "grafted NADH model" reagent In acetonitrile; benzene at 80℃; for 120h; | 100% |
| With tri(1-naphthyl)phosphonium tris(pentafluorophenyl)borohydride In dichloromethane-d2 for 12h; Reagent/catalyst; Glovebox; Sealed tube; | 100% |
-
-
102-96-5
(2-nitroethenyl)benzene

-
-
141-97-9
ethyl acetoacetate

-
-
72709-62-7
ethyl 2-acetyl-4-nitro-3-phenylbutyrate

| Conditions | Yield |
|---|---|
| With nickel polymer catalyst In chloroform for 48h; Reflux; | 100% |
| With C23H37N3O9S In dichloromethane at 20℃; for 16h; asymmetric Michael addition; enantioselective reaction; | 99% |
| With C31H36N6OS3 In toluene at 25℃; for 48h; Michael Addition; enantioselective reaction; | 99% |
-
-
102-96-5
(2-nitroethenyl)benzene

-
-
123-54-6
acetylacetone

-
-
72709-61-6
(+/-)-3-(2-nitro-1-phenylethyl)pentane-2,4-dione

| Conditions | Yield |
|---|---|
| With N,N-dimethyl-3-trimethoxysilylpropanamine:N-(3,5-bistrifluoromethylphenyl)-N'-(3-trimethoxysilyl-1-propyl)thiourea (1:1) mesoporous silica nanoparticles at 20℃; for 3h; | 100% |
| With squaramide-containing Dawson organo-polyoxotungstates In dichloromethane for 13h; Reagent/catalyst; Heating; | 99% |
| With C27H18F15N7O3 In dimethyl sulfoxide at 20℃; for 3h; Michael Addition; | 98% |
-
-
102-96-5
(2-nitroethenyl)benzene

-
-
100-53-8
phenylmethanethiol

-
-
34980-76-2, 63509-10-4, 63509-11-5
benzyl (2-nitro-1-phenylethyl) sulfide

| Conditions | Yield |
|---|---|
| at 20℃; for 0.1h; Michael addition; neat (no solvent); | 100% |
| at 20℃; for 2h; Michael addition; Neat (no solvent); regioselective reaction; | 92% |
| In water at 20℃; for 1h; thia-Michael addition; | 91% |
| With 2,2'-azobis(isobutyronitrile); quinoclamine; N,N-dimethyl-formamide; Quinine In toluene |

| Conditions | Yield |
|---|---|
| at 20℃; for 0.0166667h; Michael addition; neat (no solvent); | 100% |
| With MCM-41 immobilized phenanthrolinium dibromide In water at 20℃; for 0.5h; Catalytic behavior; Michael Addition; | 95% |
| With samarium(III) trifluoromethanesulfonate In dichloromethane at 20℃; for 24h; Reagent/catalyst; Inert atmosphere; | 94% |
-
-
102-96-5
(2-nitroethenyl)benzene

-
-
85669-65-4
methyl-4-phenylmethylamino-2-butene-1-carboxylate

| Conditions | Yield |
|---|---|
| In methanol for 16h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| at 20℃; for 0.0166667h; Michael addition; Grinding; neat (no solvent); | 100% |
| With magnesia at 25℃; for 0.3h; Green chemistry; | 98% |
| With MCM-41 immobilized phenanthrolinium dibromide In water at 20℃; for 1.5h; Catalytic behavior; Michael Addition; | 93% |
-
-
102-96-5
(2-nitroethenyl)benzene

-
-
108-94-1
cyclohexanone

-
-
4591-64-4, 51262-17-0, 51262-18-1, 84025-84-3, 84025-87-6, 84025-83-2
(S)-2-[(R)-2-nitro-1-phenylethyl]cyclohexanone

| Conditions | Yield |
|---|---|
| With 2,6-bis(1-((S)-pyrrolidin-2-ylmethyl)-1H-1,2,3-triazol-4-yl)pyridine at 20℃; for 16h; Michael addition; optical yield given as %de; | 100% |
| With C2HF3O2*C25H23N2O2PS; triethylamine; benzoic acid at -30℃; for 22h; Michael addition; stereoselective reaction; | 99% |
| Stage #1: cyclohexanone With 3-butyl-1-(butyl-4-sulfobutyl)imidazolium trifluoromethanesulfonate; C13H28N2 In water at 20℃; for 0.333333h; Michael Addition; Green chemistry; Stage #2: (2-nitroethenyl)benzene In water at 20℃; for 10h; Green chemistry; enantioselective reaction; | 99% |
-
-
102-96-5
(2-nitroethenyl)benzene

-
-
106-45-6
para-thiocresol

-
-
92851-02-0
(2-nitro-1-phenylethyl)(p-tolyl)sulfane

| Conditions | Yield |
|---|---|
| at 20℃; for 0.1h; Michael addition; Grinding; neat (no solvent); | 100% |
| In water at 20℃; for 0.166667h; thia-Michael addition; | 94% |
| With squaric acid In water at 20℃; for 1.33333h; Michael addition; | 85% |

| Conditions | Yield |
|---|---|
| With [tetrapropylammonium]2[Zn2(1,4-benzenedicarboxylate)3(dimethylamine)2] In neat (no solvent) at 70℃; for 4h; Temperature; Reagent/catalyst; Michael Addition; Green chemistry; | 100% |
| In water at 150℃; for 0.1h; Michael addition; Microwave irradiation; | 99% |
| With UiO-66(Ce) metal-organic framework In 1,2-dichloro-ethane at 80℃; for 24h; Catalytic behavior; Friedel-Crafts Alkylation; Schlenk technique; | 97% |
-
-
102-96-5
(2-nitroethenyl)benzene

-
-
1679-18-1
4-Chlorophenylboronic acid

-
-
1245819-33-3
(R)-1-chloro-4-(2-nitro-1-phenylethyl)benzene

| Conditions | Yield |
|---|---|
| With heterogeneous Rh/Ag bimetallic nanoparticle catalyst immobilized on chiral polymer In water; toluene at 100℃; for 24h; Inert atmosphere; enantioselective reaction; | 100% |
| With potassium hydrogen difluoride; C60H52Cl2Rh2 In water; toluene at 50℃; for 51h; Inert atmosphere; enantioselective reaction; | 94% |
| With chlorobis(ethylene)rhodium(I) dimer; potassium hydrogen bifluoride; C28H22 In water; toluene at 100℃; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 92% |
| With chlorobis(ethylene)rhodium(I) dimer; C17H27NO In water; toluene at 100℃; for 16h; Reagent/catalyst; Inert atmosphere; enantioselective reaction; | 87 %Chromat. |

| Conditions | Yield |
|---|---|
| at 20℃; for 0.1h; Michael addition; neat (no solvent); | 100% |
-
-
848821-58-9
(2S)-2-{diphenyl[(trimethylsilyl)oxy]methyl}pyrrolidine

-
-
102-96-5
(2-nitroethenyl)benzene

-
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| With acetic acid In toluene at 25℃; | 100% |

-
-
102-96-5
(2-nitroethenyl)benzene

-
-
1393357-24-8
5-ethyl-2-(m-tolyl)oxazol-4(5H)-one

-
-
1393357-08-8
(5R)-5-ethyl-5-((R)-2-nitro-1-phenylethyl)-2-(m-tolyl)oxazol-4(5H)-one

| Conditions | Yield |
|---|---|
| With 2,6-bis((2S-((2-naphthyl)2(HO)C)pyrrolidino)CH2)-4-Me-phenol; diethylzinc In tetrahydrofuran; hexane; propiononitrile at 20℃; for 16h; Michael reaction; Inert atmosphere; optical yield given as %ee; stereoselective reaction; | 100% |
-
-
102-96-5
(2-nitroethenyl)benzene

-
-
1393357-25-9
5-isobutyl-2-(m-tolyl)oxazol-4(5H)-one

| Conditions | Yield |
|---|---|
| With 2,6-bis((2S-((2-naphthyl)2(HO)C)pyrrolidino)CH2)-4-Me-phenol; diethylzinc In tetrahydrofuran; hexane; propiononitrile at 20℃; for 16h; Michael reaction; Inert atmosphere; optical yield given as %ee; stereoselective reaction; | 100% |
-
-
102-96-5
(2-nitroethenyl)benzene

-
-
1393357-27-1
5-allyl-2-(m-tolyl)oxazol-4(5H)-one

-
-
1393357-11-3
(5R)-5-allyl-5-((R)-2-nitro-1-phenylethyl)-2-(m-tolyl)oxazol-4(5H)-one

| Conditions | Yield |
|---|---|
| With 2,6-bis((2S-((2-naphthyl)2(HO)C)pyrrolidino)CH2)-4-Me-phenol; diethylzinc In tetrahydrofuran; hexane; propiononitrile at 20℃; for 16h; Michael reaction; Inert atmosphere; optical yield given as %ee; stereoselective reaction; | 100% |
-
-
102-96-5
(2-nitroethenyl)benzene

-
-
1393357-29-3
5-(2-(methylthio)ethyl)-2-(m-tolyl)oxazol-4(5H)-one

-
-
1393357-13-5
(5R)-5-(2-(methylthio)ethyl)-5-((R)-2-nitro-1-phenylethyl)-2-(m-tolyl)oxazol-4(5H)-one

| Conditions | Yield |
|---|---|
| With 2,6-bis((2S-((2-naphthyl)2(HO)C)pyrrolidino)CH2)-4-Me-phenol; diethylzinc In tetrahydrofuran; hexane; propiononitrile at 20℃; for 16h; Michael reaction; Inert atmosphere; optical yield given as %ee; stereoselective reaction; | 100% |
-
-
102-96-5
(2-nitroethenyl)benzene

| Conditions | Yield |
|---|---|
| In water at 20℃; for 8h; Solvent; regioselective reaction; | 100% |
-
-
39718-00-8
2-chlorobenzylthiol

-
-
102-96-5
(2-nitroethenyl)benzene

| Conditions | Yield |
|---|---|
| Stage #1: (2-nitroethenyl)benzene With 1-[(3R,4S)-1-benzyl-4-phenylpyrrolidin-3-yl]-3-[3,5-bis(trifluoromethyl)phenyl]urea In dichloromethane at 20℃; for 0.333333h; Molecular sieve; Stage #2: 2-chlorobenzylthiol In dichloromethane at -80℃; for 48h; Michael Addition; Molecular sieve; enantioselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| With 2,2,2-trifluoroethanol; 1-(3,5-bis(trifluoromethyl)phenyl)-3-(2-(difluoroboryl)phenyl)urea In dichloromethane at 23℃; for 24h; Kinetics; Reagent/catalyst; Inert atmosphere; | 99% |
| Stage #1: (2-nitroethenyl)benzene With C24H12F8N2O7Si2 In 1,2-dichloro-benzene for 0.0833333h; Inert atmosphere; Stage #2: indole In 1,2-dichloro-benzene at 23℃; for 24h; Reagent/catalyst; Solvent; Inert atmosphere; | 99% |
| With zinc diacetate In ethanol at 20℃; for 0.333333h; Michael addition; | 98% |

| Conditions | Yield |
|---|---|
| at 20℃; for 0.116667h; Michael addition; neat (no solvent); | 99% |
| With magnesia at 25℃; for 0.4h; Green chemistry; | 95% |
| With iranian dolomite In water at 20℃; for 0.5h; Michael Addition; | 93% |
-
-
102-96-5
(2-nitroethenyl)benzene

-
-
91-60-1
2-Naphthalenethiol

-
-
110130-43-3
[2]naphthyl-(2-nitro-1-phenyl-ethyl)-sulfide

| Conditions | Yield |
|---|---|
| at 20℃; for 0.116667h; Michael addition; Grinding; neat (no solvent); | 99% |
| With diethyl ether; triethylamine |
-
-
102-96-5
(2-nitroethenyl)benzene

-
-
507-09-5
thioacetic acid

-
-
29651-81-8
S-2-nitro-1-phenylethyl thiolacetate

| Conditions | Yield |
|---|---|
| With tributyl-amine In diethyl ether for 1h; Michael addition; Cooling with ice; | 99% |
| at 20℃; for 0.0833333h; Michael condensation; neat (no solvent); | 98% |
| With piperidine |
-
-
102-96-5
(2-nitroethenyl)benzene

-
-
93-91-4
1-phenylbutan-1,3-dione

-
-
82947-13-5
2-(2-nitro-1-phenylethyl)-1-phenylbutane-1,3-dione

| Conditions | Yield |
|---|---|
| With squaramide-containing Dawson organo-polyoxotungstates In dichloromethane for 13h; Reagent/catalyst; Heating; | 99% |
| at 20℃; Michael addition; Neat (no solvent); Grinding; optical yield given as %de; | 70% |
| bis(acetylacetonate)nickel(II) In 1,4-dioxane for 46h; Heating; | 60% |
| With triethylamine In dichloromethane at 20℃; for 24h; Michael Addition; |
-
-
102-96-5
(2-nitroethenyl)benzene

-
-
2365-48-2
Methyl thioglycolate

-
-
76665-83-3, 91134-32-6
methyl {[alpha-(nitromethyl)benzyl]thio}acetate

| Conditions | Yield |
|---|---|
| With C37H56N4O4; lanthanum(lll) triflate In 1,2-dichloro-ethane at 0℃; for 1h; Sulfa-Michael addition; | 99% |
| With triethylamine In tetrahydrofuran at 20℃; for 2h; Michael addition; | 97% |
| In benzene Heating; |
-
-
102-96-5
(2-nitroethenyl)benzene

-
-
108-88-3
toluene

-
-
4099-50-7
N-hydroxy-2-phenyl-2-p-tolylacetimidoyl chloride

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In dichloromethane at 0 - 20℃; Friedel-Crafts alkylation; Inert atmosphere; | 99% |
| With aluminium trichloride |
-
-
102-96-5
(2-nitroethenyl)benzene

-
-
1099-45-2
ethyl (triphenylphosphoranylidene)acetate

-
-
37709-90-3
4-Nitro-3-phenyl-2-(triphenyl-λ5-phosphanylidene)-butyric acid ethyl ester

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 3h; Michael condensation; | 99% |
-
-
102-96-5
(2-nitroethenyl)benzene

-
-
108-98-5
thiophenol

-
-
4231-84-9
(2-nitro-1-phenylethyl) phenyl sulfide

| Conditions | Yield |
|---|---|
| With C23H32F6N4O2S2 In dichloromethane at -40℃; for 1h; Michael Addition; | 99% |
| at 20℃; for 0.116667h; Michael addition; neat (no solvent); | 98% |
| With triethylamine In ethanol at 20℃; for 0.0833333h; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With di(naphthalen-1-yl)silanediol In dichloromethane at 23℃; for 48h; Inert atmosphere; | 99% |
| With 2,6-bis(2,2-dimethylpropionylamino)benzoic acid In chloroform at 40℃; for 24h; Friedel-Crafts Alkylation; | 99% |
| With 1,1,1,3',3',3'-hexafluoro-propanol at 20℃; for 2h; Friedel-Crafts Alkylation; | 96% |
β-NITROSTYRENE Chemical Properties
IUPAC Name: 1-Nitro-2-phenylethylene
The MF of 1-Nitro-2-phenylethylene (102-96-5) is C8H7NO2.
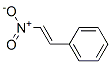
The MW of 1-Nitro-2-phenylethylene (102-96-5) is 149.15.
Synonyms of 1-Nitro-2-phenylethylene (102-96-5): (2-Nitroethenyl)benzene ; trans-beta-Nitrostyrene ; .beta.-Nitrostyrene ; beta-nitrostyrene ; Styrene and beta-nitrostyrene mixture ; Styrene, .beta.-nitro- ; Styrene, .beta.-nitro-, (E)-
Stability: Stable. Combustible. Incompatible with strong oxidizing agents, strong bases. May be sensitive to prolonged exposure to air
Form: Yellow prisms (from ethanol) or yellow crystalline solid
Index of Refraction: 1.603
EINECS: 203-066-0
Density: 1.177 g/ml
Flash Point: 117.5 °C
Boiling Point: 255.8 °C
Melting Point: 55-58 °C
Storage temp: 2-8 °C
β-NITROSTYRENE Toxicity Data With Reference
| 1. | orl-mus LDLo:710 mg/kg | AECTCV Archives of Environmental Contamination and Toxicology. 14 (1985),111. |
β-NITROSTYRENE Consensus Reports
Reported in EPA TSCA Inventory.
β-NITROSTYRENE Safety Profile
Moderately toxic by ingestion. When heated to decomposition it emits toxic vapors of NOx.Safety information of 1-Nitro-2-phenylethylene (102-96-5):
Hazard Codes ![]() Xi
Xi
Risk Statements
36/37/38 Irritating to eyes, respiratory system and skin
Safety Statements
26 In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36 Wear suitable protective clothing
WGK Germany 3
RTECS WL5460000
Related Products
- β-NITROSTYRENE
- 1029654-24-7
- 1029654-27-0
- 1029684-36-3
- 1029684-37-4
- 10297-05-9
- 10297-06-0
- 10297-57-1
- 102-97-6
- 10297-73-1
- 1029872-54-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View