-
Name
1,2-Diphenylhydrazine
- EINECS 204-563-5
- CAS No. 122-66-7
- Article Data310
- CAS DataBase
- Density 1.179 g/cm3
- Solubility 221 mg/L at 25 °C (U.S. EPA, 1980a)
- Melting Point 123-126 °C(lit.)
- Formula C12H12N2
- Boiling Point 229.3 °C at 760 mmHg
- Molecular Weight 184.241
- Flash Point 87.7 °C
- Transport Information UN 3077 9/PG 3
- Appearance yellow crystalline powder
- Safety 53-45-60-61
- Risk Codes 45-22-50/53
-
Molecular Structure
-
Hazard Symbols
 T,
T,  N
N
- Synonyms Hydrazobenzene(8CI);Benzene,1,1'-hydrazobis-;N,N'-Bianiline;N,N'-Diphenylhydrazine;
- PSA 24.06000
- LogP 3.27160
Synthetic route

| Conditions | Yield |
|---|---|
| With ammonia borane; gold on titanium oxide In ethanol at 20℃; for 0.5h; Inert atmosphere; | A 92% B 100% |
| With samarium; iodine; ammonium chloride In tetrahydrofuran; water at 20℃; for 4h; Reduction; | A 56% B 20% |
| Electrolysis; |

-
-
122-66-7
diphenyl hydrazine

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In chloroform-d1; water at 20℃; for 0.0833333h; | 100% |
-
-
1227476-15-4
Azobenzene

-
-
122-66-7
diphenyl hydrazine

| Conditions | Yield |
|---|---|
| With borane-ammonia complex; [C6H3-2,6-(CHN(t-Bu))2]Bi; water In tetrahydrofuran at 35℃; for 2h; Mechanism; Reagent/catalyst; Solvent; Temperature; Schlenk technique; Inert atmosphere; | 99% |
| With ammonium formate; 1-butyl-3-methylimidazolium Tetrafluoroborate; zinc In water at 20℃; for 0.416667h; | 98% |
| With hydrogen; potassium hydroxide In para-xylene at 80℃; for 5h; | 98.1% |

| Conditions | Yield |
|---|---|
| With sodium hydrogensulfide In ammonia at 40℃; for 5h; Product distribution; | 99% |
| With hydrazine hydrate; sodium hydroxide; [1,4]naphthoquinone at 55℃; for 6h; Temperature; | 99% |
| With sodium dithionite; 1,1′-dioctyl-4,4′-bipyridinium; potassium carbonate In water; acetonitrile at 35℃; for 1h; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: Azobenzene; bis(pinacol)diborane With pyridine-4-carbonitrile In pentane at 70℃; for 24h; Inert atmosphere; Stage #2: With water In pentane at 20℃; for 0.25h; Concentration; Temperature; | 99% |

| Conditions | Yield |
|---|---|
| With aluminum telluride; water In tetrahydrofuran for 0.333333h; Product distribution; Heating; | A 98% B 1% |
| With aluminum telluride; water In tetrahydrofuran for 0.333333h; Heating; | A 98% B 1% |
| With bis(1,5-cyclooctadiene)diiridium(I) dichloride; 1,10-Phenanthroline; isopropyl alcohol; potassium hydroxide at 83℃; for 2h; Schlenk technique; Inert atmosphere; | A 89 %Chromat. B 11 %Chromat. |

| Conditions | Yield |
|---|---|
| With triethylsilane; indium(III) bromide In N,N-dimethyl-formamide at 60℃; for 8h; | 96% |
| With ammonia; lithium perchlorate In acetonitrile at 20℃; Electrolysis; chemoselective reaction; | 96% |
| With potassium hydroxide; aluminium In methanol at 20℃; for 0.25h; Reduction; Reductive coupling; | 95% |

| Conditions | Yield |
|---|---|
| With aluminum telluride; water In tetrahydrofuran for 0.333333h; Heating; | A 4% B 96% |
| With aluminum telluride; water In tetrahydrofuran for 0.333333h; Product distribution; Heating; | A 4% B 96% |
| With lithium vanadium(I) dihydride In tetrahydrofuran at 25℃; for 12h; Inert atmosphere; | A 15% B 85% |

| Conditions | Yield |
|---|---|
| With triethylsilane; indium(III) bromide In N,N-dimethyl-formamide at 60℃; | 94% |
| With diethyl ether; magnesium; magnesium iodide weiteres Reagens: Benzol; Eintragen des Reaktionsgemisches in Wasser; | |
| Multi-step reaction with 3 steps 1: hydrazine 2: ; oxygen / ethanol / 1 h / 60 °C 3: ; hydrazine / 20 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: acetic acid / ethanol / 20 °C / Inert atmosphere 2: potassium carbonate; C30H29BrMnNO2P2; hydrogen / toluene / 24 h / 135 °C / 60006 Torr / Glovebox; Autoclave View Scheme |
-
-
17082-12-1
trans-azobenzene

-
-
122-66-7
diphenyl hydrazine

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol at 60℃; for 0.5h; | 92% |
| With borane-ammonia complex; tris(quinolin-8-yl)phosphite In tetrahydrofuran at 60℃; Kinetics; Catalytic behavior; Reagent/catalyst; Schlenk technique; Inert atmosphere; Sealed tube; | 91% |
| With sodium hydrogencarbonate; hydrazine hydrate; zirconium(IV) oxide In ethanol; water for 10h; Heating; | 86% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; platinum/charcoal In n-heptane; water; hydrogen | 91.5% |

-
-
122-66-7
diphenyl hydrazine

| Conditions | Yield |
|---|---|
| With water at 20℃; for 48h; Inert atmosphere; | 90% |
| With water at 20℃; for 0.166667h; Inert atmosphere; |
-
-
782-74-1
1,2-bis(2-chlorophenyl)hydrazine

-
-
71-36-3
butan-1-ol

-
A
-
95-51-2
2-Chloroaniline

-
B
-
122-66-7
diphenyl hydrazine

| Conditions | Yield |
|---|---|
| A n/a B 89.8% |


| Conditions | Yield |
|---|---|
| With aluminum telluride; water In tetrahydrofuran for 0.333333h; Heating; | A 6% B 86% |
| With aluminum telluride; water In tetrahydrofuran for 0.333333h; Product distribution; Heating; | A 6% B 86% |
-
-
1227476-15-4
Azobenzene

-
-
100-51-6
benzyl alcohol

-
A
-
758640-21-0
N-Benzylaniline

-
B
-
538-51-2
benzylidene phenylamine

-
C
-
122-66-7
diphenyl hydrazine

| Conditions | Yield |
|---|---|
| With 1 wtpercent of Pd on TiO2 In water at 29.84℃; for 4h; UV-irradiation; Inert atmosphere; | A 85% B 5% C 10% |


-
-
122-66-7
diphenyl hydrazine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In isopropyl alcohol; toluene at 20℃; for 22h; Inert atmosphere; | 85% |
-
-
867-13-0
diethoxyphosphoryl-acetic acid ethyl ester

-
-
123-11-5
4-methoxy-benzaldehyde

-
-
1227476-15-4
Azobenzene

-
A
-
24393-56-4
ethyl (E)-3-(4-methoxyphenyl)prop-2-enoate

-
B
-
122-66-7
diphenyl hydrazine

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide Ambient temperature; electrolysis; | A 80% B n/a |


| Conditions | Yield |
|---|---|
| With 1,3-bis(N',N'-dimethyl-4-aminopyridinium)propane diiodide; sodium hydride In N,N-dimethyl-formamide at 20℃; for 18h; UV-irradiation; | A 5% B 80% |
| With 1,3-bis(N',N'-dimethyl-4-aminopyridinium)propane diiodide; sodium hydride In N,N-dimethyl-formamide at 20℃; for 18h; UV-irradiation; | A 73% B 18% |
| With sulfur In various solvent(s) at 100℃; for 2h; in an autoclave; | A 84 % Chromat. B 12 % Chromat. |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; aluminium In methanol at 20℃; for 0.416667h; Reduction; Reductive coupling; | A 14% B 80% |
-
-
88284-48-4
2-(trimethylsilyl)phenyl trifluoromethanesulfonate

-
-
1576-35-8
toluene-4-sulfonic acid hydrazide

-
A
-
640-57-3
phenyltolylsulfone

-
B
-
122-66-7
diphenyl hydrazine

| Conditions | Yield |
|---|---|
| With cesium fluoride In acetonitrile at 90℃; | A 75% B 12% |

-
-
3699-66-9
ethyl 2-diethoxyphosphorylpropionate

-
-
123-11-5
4-methoxy-benzaldehyde

-
-
1227476-15-4
Azobenzene

-
A
-
52750-05-7, 119870-81-4, 115754-00-2
ethyl (E)-3-(4-methoxyphenyl)-2-methylpropenoate

-
B
-
122-66-7
diphenyl hydrazine

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide Ambient temperature; electrolysis; | A 70% B n/a |

-
-
3167-62-2
diethyl (dichloromethyl)phosphonate

-
-
123-11-5
4-methoxy-benzaldehyde

-
-
1227476-15-4
Azobenzene

-
A
-
41448-64-0
1-(2,2-dichlorovinyl)-4-methoxybenzene

-
B
-
122-66-7
diphenyl hydrazine

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide Ambient temperature; electrolysis; | A 70% B n/a |

-
-
98-95-3
nitrobenzene

-
A
-
62-53-3
aniline

-
B
-
122-66-7
diphenyl hydrazine

-
C
-
1227476-15-4
Azobenzene

-
D
-
495-48-7
azoxybenzene

| Conditions | Yield |
|---|---|
| With indium; iodine In methanol at 50℃; for 12h; Product distribution; Further Variations:; reagents molar ratio; | A 69% B 20% C n/a D n/a |

-
-
20972-43-4, 21650-65-7, 495-48-7
trans-azoxybenzene

-
A
-
122-66-7
diphenyl hydrazine

-
B
-
17082-12-1
trans-azobenzene

| Conditions | Yield |
|---|---|
| With 2-hydroxyethanethiol; {Fe(III)2Mn(II)O(OAc)6(Py)3} In N,N-dimethyl-formamide at 70℃; for 40h; | A 66% B 5% |

| Conditions | Yield |
|---|---|
| With indium(III) triflate; triethylsilane In N,N-dimethyl-formamide at 60℃; for 12h; Inert atmosphere; | A 29% B 64% |
| With 1,3-bis(N',N'-dimethyl-4-aminopyridinium)propane diiodide; sodium hydride In N,N-dimethyl-formamide at 20℃; for 18h; UV-irradiation; | A 59% B 23% |
| With methanol; iodine; magnesium for 1h; Product distribution; Ambient temperature; other nitroarenes, var. time, var. molar ratio, var. alcohols, also oxygen; | A 34.5 % Chromat. B 65.4 % Chromat. |

| Conditions | Yield |
|---|---|
| With aluminum telluride; water In tetrahydrofuran for 0.333333h; Product distribution; Heating; | A 16% B 63% |
| With aluminum telluride; water In tetrahydrofuran for 0.333333h; Heating; | A 16% B 63% |
-
-
1080-32-6
O,O-diethyl benzylphosphonate

-
-
123-11-5
4-methoxy-benzaldehyde

-
-
1227476-15-4
Azobenzene

-
A
-
1694-19-5
E-1-(phenyl)-2-(4'-methoxyphenyl)ethene

-
B
-
122-66-7
diphenyl hydrazine

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide Ambient temperature; electrolysis; | A 60% B n/a |

-
-
1080-32-6
O,O-diethyl benzylphosphonate

-
-
100-10-7
4-dimethylamino-benzaldehyde

-
-
1227476-15-4
Azobenzene

-
A
-
838-95-9
(E)-N,N-dimethyl-4-styrylbenzenamine

-
B
-
122-66-7
diphenyl hydrazine

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide Ambient temperature; electrolysis; | A 55% B n/a |

-
-
1227476-15-4
Azobenzene

-
-
100-51-6
benzyl alcohol

-
A
-
758640-21-0
N-Benzylaniline

-
B
-
538-51-2
benzylidene phenylamine

-
C
-
62-53-3
aniline

-
D
-
122-66-7
diphenyl hydrazine

| Conditions | Yield |
|---|---|
| With 0.5 wtpercent of Pd on TiO2 In water at 29.84℃; for 4h; UV-irradiation; Inert atmosphere; | A 47% B 10% C 10% D 27% |
| With 2 wtpercent of Pd on TiO2 In water at 29.84℃; for 4h; UV-irradiation; Inert atmosphere; | A 6% B 24% C 30% D 30% |


| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; nickel dichloride In methanol for 15h; Ambient temperature; | A 17% B 12.1% C 45.7% |
| With sodium tetrahydroborate; chloro(1,5-cyclooctadiene)rhodium(I) dimer In isopropyl alcohol at 82.4℃; |

-
-
122-66-7
diphenyl hydrazine

-
-
1227476-15-4
Azobenzene

| Conditions | Yield |
|---|---|
| With sodium periodate In dichloromethane at 20℃; for 1h; | 100% |
| With pyridine; bromine In dichloromethane at 0℃; for 0.25h; | 100% |
| With benzeneseleninic anhydride In tetrahydrofuran for 0.166667h; Ambient temperature; | 99% |
-
-
122-66-7
diphenyl hydrazine

-
-
17082-12-1
trans-azobenzene

| Conditions | Yield |
|---|---|
| With tert-butylhypochlorite; sodium iodide In acetonitrile at 20℃; for 1h; Inert atmosphere; | 100% |
| With potassium tert-butylate; ammonia at -75 - 20℃; for 0.05h; Reagent/catalyst; Temperature; Sealed tube; | 99% |
| With pyridine; oxygen; copper(I) bromide In toluene under 760.051 Torr; for 0.25h; | 98% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 48h; | 98% |
-
-
67605-92-9
dimethylbis(η5-pentamethylcyclopentadienyl)uranium

-
-
122-66-7
diphenyl hydrazine

| Conditions | Yield |
|---|---|
| With C6Me6 In diethyl ether (Ar or He); stirring (68 h); recrystn. (toluene/diethyl ether); | 98% |
-
-
4559-70-0
Diphenylphosphine oxide

-
-
122-66-7
diphenyl hydrazine

-
-
64718-16-7
N,N′,P,P-tetraphenylphosphinic hydrazide

| Conditions | Yield |
|---|---|
| With caesium carbonate In acetonitrile at 100℃; for 0.5h; Sealed tube; | 98% |

| Conditions | Yield |
|---|---|
| With ammonium acetate In ethanol for 0.0333333h; Reflux; Green chemistry; | 98% |

-
-
63976-76-1
1,1'-thiocarbonylbis(1,2,4-triazole)

-
-
122-66-7
diphenyl hydrazine

-
-
90396-11-5
[1,2,4]Triazole-1-carbothioic acid N,N'-diphenyl-hydrazide

| Conditions | Yield |
|---|---|
| In acetone for 1.5h; Heating; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: diphenyl hydrazine; methyl iodide With n-butyllithium In tetrahydrofuran; hexane at -60 - 20℃; for 2h; Stage #2: methyl iodide In tetrahydrofuran; hexane for 3h; Further stages.; | 97% |
| Stage #1: diphenyl hydrazine With n-butyllithium In tetrahydrofuran; hexane at -78 - -60℃; for 0.25h; Inert atmosphere; Stage #2: methyl iodide In tetrahydrofuran; hexane at -60 - 20℃; for 4h; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: diphenyl hydrazine With trimethylsilylmethyllithium In tetrahydrofuran at 20℃; for 4h; Inert atmosphere; Stage #2: tetrahydrofuran In hexane at -20℃; Inert atmosphere; | 97% |
-
-
15754-51-5
bis(4-methoxyphenyl)phosphine oxide

-
-
122-66-7
diphenyl hydrazine

| Conditions | Yield |
|---|---|
| With caesium carbonate In acetonitrile at 100℃; for 1h; Sealed tube; | 97% |

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane at -60 - 20℃; for 1h; | 96% |
| Stage #1: diphenyl hydrazine With n-butyllithium In tetrahydrofuran; hexane at -78 - -60℃; for 0.25h; Inert atmosphere; Stage #2: allyl bromide In tetrahydrofuran; hexane at -60 - 20℃; for 1h; Inert atmosphere; | 96% |
| With tetra(n-butyl)ammonium hydroxide; potassium bromide In dichloromethane |

| Conditions | Yield |
|---|---|
| With ammonium acetate In ethanol for 0.5h; Reflux; Green chemistry; | 96% |

-
-
4755-77-5
Ethyl oxalyl chloride

-
-
122-66-7
diphenyl hydrazine

-
-
97738-62-0
ethyl 2-(1,2-diphenylhydrazino)-2-oxoacetate

| Conditions | Yield |
|---|---|
| With triethylamine | 95% |

| Conditions | Yield |
|---|---|
| With ammonium acetate In ethanol for 0.5h; Reflux; Green chemistry; | 95% |


| Conditions | Yield |
|---|---|
| With hydrogen; NiCl2-Li-[poly(2-vinyl-naphthalene)-co-(divinylbenzene)] In tetrahydrofuran at 20℃; under 760.051 Torr; for 12h; | 93% |
| With titanium tetrachloride; magnesium In tetrahydrofuran at 20℃; Inert atmosphere; | 91% |
| With β-D-glucose; water at 25℃; for 10h; UV-irradiation; | 90% |
-
-
538-75-0
dicyclohexyl-carbodiimide

-
-
122-66-7
diphenyl hydrazine

-
A
-
4833-42-5
N-phenyl-N',N-dicyclohexylguanidine

-
B
-
17082-12-1
trans-azobenzene

| Conditions | Yield |
|---|---|
| With trimethylsilylmethyllithium In benzene at 110℃; for 24h; Glovebox; Schlenk technique; Inert atmosphere; | A 93% B n/a |


-
-
122-66-7
diphenyl hydrazine

| Conditions | Yield |
|---|---|
| With caesium carbonate In acetonitrile at 100℃; for 0.5h; Reagent/catalyst; Solvent; Temperature; Sealed tube; | 93% |

| Conditions | Yield |
|---|---|
| With ammonium acetate In ethanol for 0.5h; Reflux; Green chemistry; | 93% |

-
-
873-51-8
phenylborondichloride

-
-
122-66-7
diphenyl hydrazine

-
-
13831-68-0
hexaphenyl-1.2.4.5-tetraaza-3.6-diborane

| Conditions | Yield |
|---|---|
| With triethylamine In benzene 4 h heating 40-60°C; | 92% |
| With (C2H5)3N In benzene 4 h heating 40-60°C; | 92% |
| With triethylamine In benzene |
-
-
99910-01-7
2-(p-methoxyphenyl)-4-phenyl-6-(p-nitrophenyl)-3,4-dihydro-2H-sym-tetrazin-1-yl

-
-
122-66-7
diphenyl hydrazine

-
A
-
96385-57-8
2-(p-methoxyphenyl)-4-phenyl-6-(p-nitrophenyl)-1,2,3,4-tetrahydro-sym-tetrazine

-
B
-
1227476-15-4
Azobenzene

| Conditions | Yield |
|---|---|
| In benzene | A 92% B 90% |
| In acetonitrile at 20℃; Rate constant; Equilibrium constant; activation energy (Ea), ΔS(excit.); |

1,2-Diphenylhydrazine Consensus Reports
NTP 10th Report on Carcinogens. NCI Carcinogenesis Bioassay (feed); Clear Evidence: mouse, rat NCITR* National Cancer Institute Carcinogenesis Technical Report Series. (Bethesda, MD 20014) No. NCI-CG-TR-92 ,1978. . Community Right-To-Know List. Reported in EPA TSCA Inventory.
1,2-Diphenylhydrazine Standards and Recommendations
DFG MAK: Animal Carcinogen, Suspected Human Carcinogen
1,2-Diphenylhydrazine Specification
The Hydrazobenzene with CAS registry number of 122-66-7 is also called Hydrazine,1,2-diphenyl-. The IUPAC name is 1,2-diphenylhydrazine. Its EINECS registry number is 204-563-5. In addition, the molecular formula is C12H12N2 and the molecular weight is 184.24. It is a kind of yellow crystalline powder.
Physical properties about this chemical are: (1)ACD/LogP: 2.94; (2)ACD/LogD (pH 5.5): 2.94; (3)ACD/LogD (pH 7.4): 2.94; (4)ACD/BCF (pH 5.5): 100.69; (5)ACD/BCF (pH 7.4): 101.01; (6)ACD/KOC (pH 5.5): 943.92; (7)ACD/KOC (pH 7.4): 946.98; (8)#H bond acceptors: 2; (9)#H bond donors: 2; (10)#Freely Rotating Bonds: 3; (11)Polar Surface Area: 6.48 Å2; (12)Index of Refraction: 1.7; (13)Molar Refractivity: 60.4 cm3; (14)Molar Volume: 156.2 cm3; (15)Polarizability: 23.94 ×10-24cm3; (16)Surface Tension: 52.5 dyne/cm; (17)Density: 1.179 g/cm3; (18)Flash Point: 87.7 °C; (19)Enthalpy of Vaporization: 46.59 kJ/mol; (20)Boiling Point: 229.3 °C at 760 mmHg; (21)Vapour Pressure: 0.07 mmHg at 25°C.
Preparation of Hydrazobenzene: it can be prepared by diphenyldiazene. This reaction is a kind of reduction reaction. And it will need reagents ultrasonically activated nickel and N2H4*H2O, and solvents toluene and tetrahydrofuran. The reaction time is 5 minutes at reaction temperature of 20 °C. The yield is about 90.3%.

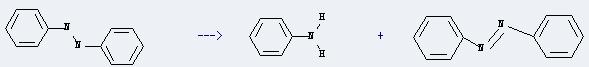
Uses of Hydrazobenzene: this chemical can be used to produce anti-inflammatory drugs in pharmaceutical industry and benzidine dyes. In addition, it can be used to get trans-diphenyldiazene and aniline. This reaction will need reagent hydrated HZSM-5. The reaction time is 30 minutes at reaction temperature of 150 °C. The yield is about 59%.
When you are using this chemical, please be cautious about it as the following:
This chemical is harmful if swallowed and may cause cancer. In addition, it is very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. You should avoid exposure-obtain special instructions before use. During using it, you should avoid release to the environment. And you can refer to special instructions/safety data sheets. In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.).
You can still convert the following datas into molecular structure:
(1)SMILES: c2(NNc1ccccc1)ccccc2
(2)InChI: InChI=1/C12H12N2/c1-3-7-11(8-4-1)13-14-12-9-5-2-6-10-12/h1-10,13-14H
(3)InChIKey: YBQZXXMEJHZYMB-UHFFFAOYAR
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 1226-71-7
- 122672-46-2
- 1226776-92-6
- 1226776-95-9
- 122-67-8
- 122684-33-7
- 122684-34-8
- 122684-35-9
- 122-68-9
- 122-69-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View