-
Name
1-Naphthalene acetic acid
- EINECS 201-705-8
- CAS No. 86-87-3
- Article Data96
- CAS DataBase
- Density 1.263 g/cm3
- Solubility Soluble in diethyl ether, acetone, chloroform and acetic acid. Slightly soluble in cold water and ethanol
- Melting Point 126-133.5 oC
- Formula C12H10O2
- Boiling Point 373.207 °C at 760 mmHg
- Molecular Weight 186.21
- Flash Point 270.1 °C
- Transport Information
- Appearance white to off white crystals
- Safety 26-24/25-22-23-45-36/37/39-27
- Risk Codes 36/37/38-22-68-41-34
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, Xn,
Xn, C
C
- Synonyms 1-NAA;1-Naphthylacetic acid;2-(1-Naphthyl)acetic acid;2-(Naphthalen-1-yl)aceticacid;2-(a-Naphthyl)ethanoic acid;ANU;Agronaa;Alman;Biokor;Celmone;Etifix;Fruitofix;Fruitone N;Germon;N 10;N40;NAA;NSC 15772;Naftal;Nafusaku;Naphthalen-1-ylacetic acid;Naphthaleneacetic acid;Naphthylacetic acid;Phyomone;Planofix;Planofixe;Pomoxon;Raizon 05;Rasin;Rhizopon B;Rhodofix;Stimolante 66f;Tre-hold;Vardhak;a-NAA;a-Naphthaleneacetic acid;a-Naphthylacetic acid;
- PSA 37.30000
- LogP 2.46690
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: 1-naphthylmethyl halide With lithium chloride; zinc Stage #2: carbon dioxide With lithium chloride In N,N-dimethyl-formamide at 50℃; under 760.051 Torr; for 24h; Stage #3: With hydrogenchloride; water In N,N-dimethyl-formamide | 100% |

| Conditions | Yield |
|---|---|
| With periodic acid; tripropylammonium fluorochromate (VI) In acetonitrile at 0℃; | 99% |
| With periodic acid; pyridinium chlorochromate In acetonitrile | 97% |

| Conditions | Yield |
|---|---|
| at 200℃; Molecular sieve; Large scale; | 96.7% |
| at 165℃; dann auf 180-185grad; | |
| With iron; potassium bromide at 220℃; |
-
-
91668-57-4
α-(N-Methylanilino)-β-(1-naphthyl)acrylonitrile

-
-
86-87-3
naphth-1-yl acetic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran for 3h; Heating; | 96% |
-
-
86803-54-5
(Z)-α-(N-Methylanilino)-β-(1-naphthyl)acrylonitrile

-
-
86-87-3
naphth-1-yl acetic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran for 3h; Heating; | 96% |

-
-
86-87-3
naphth-1-yl acetic acid

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane at 20℃; for 0.0833333h; Reagent/catalyst; | 94% |

| Conditions | Yield |
|---|---|
| With high-silica Hβ-75 zeolite In water at 130℃; for 24h; | 93% |
| With potassium carbonate; thiophenol In 1-methyl-pyrrolidin-2-one at 190℃; for 0.166667h; Substitution; | 90% |
| With potassium fluoride; thiophenol In various solvent(s) at 190℃; for 0.166667h; | 80% |
-
-
119670-67-6
Naphthalen-1-yl-acetic acid 2-oxo-2-phenyl-ethyl ester

-
-
86-87-3
naphth-1-yl acetic acid

| Conditions | Yield |
|---|---|
| With sodium hydrogen telluride In N,N-dimethyl-formamide for 0.333333h; Ambient temperature; | 93% |

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide In 1,4-dioxane at 60℃; for 2h; pH=10 - 14; | 92% |
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| With potassium phosphate buffer at 30℃; for 96h; Rhodococcus sp. AJ270 cells; | 91.3% |
| Stage #1: naphthalen-1-ylacetonitrile With sodium hydroxide In water for 3h; Reflux; Stage #2: With hydrogenchloride In water pH=2; Time; | 74.8% |
| With sulfuric acid; acetic acid |
-
-
43017-75-0
1-naphthylacetaldehyde

-
-
86-87-3
naphth-1-yl acetic acid

| Conditions | Yield |
|---|---|
| With N-hydroxyphthalimide; oxygen In acetonitrile at 60℃; for 3h; Schlenk technique; | 87% |
| With N-hydroxyphthalimide; oxygen In acetonitrile at 30℃; under 760.051 Torr; for 3h; Schlenk technique; | 87% |
| With AgOH |

| Conditions | Yield |
|---|---|
| Stage #1: carbon monoxide With C28H22CoN4O6 In butan-1-ol at 60℃; under 760.051 Torr; for 2h; Glovebox; High pressure; Green chemistry; Stage #2: 1-Chloromethylnaphthalene With tetra-(n-butyl)ammonium iodide; sodium hydroxide In butan-1-ol at 60℃; under 760.051 Torr; for 22h; Glovebox; High pressure; Green chemistry; regioselective reaction; | 85% |
| With sodium hydroxide; cerium(III) chloride; cetyltrimethylammonim bromide; nickel cyanide In toluene at 85 - 95℃; under 1 Torr; for 7h; | 60% |
| With water; palladium diacetate at 110℃; under 37503.8 Torr; for 4h; Ionic liquid; | 92 %Chromat. |
-
-
89244-21-3, 89244-22-4
N-((E)-1-Cyano-2-naphthalen-1-yl-vinyl)-4,N-dimethyl-benzamide

-
-
86-87-3
naphth-1-yl acetic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran for 6h; Heating; | 83% |
-
-
33656-65-4
ethyl 2-(1-naphthyl)-2-oxoacetate

-
-
86-87-3
naphth-1-yl acetic acid

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-(1-naphthyl)-2-oxoacetate With hydrazine In 1,2-dimethoxyethane at 130℃; for 2h; Stage #2: With potassium hydroxide In 1,2-dimethoxyethane at 80℃; for 8h; Temperature; Solvent; Reflux; | 83% |

| Conditions | Yield |
|---|---|
| With niobium(V) oxide; water In neat (no solvent) for 30h; Reflux; Inert atmosphere; | 83% |
-
-
124-38-9
carbon dioxide

-
-
16430-29-8
naphthalen-1-ylmethylene-hydrazone

-
-
86-87-3
naphth-1-yl acetic acid

| Conditions | Yield |
|---|---|
| With 1,1'-bis-(diphenylphosphino)ferrocene; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; caesium carbonate; cesium fluoride In N,N-dimethyl-formamide at 80℃; under 760.051 Torr; for 24h; chemoselective reaction; | 81% |
-
-
67-56-1
methanol

-
-
114498-69-0
Naphthalen-1-yl-acetic acid pyren-1-ylmethyl ester

-
A
-
86-87-3
naphth-1-yl acetic acid

-
B
-
91385-15-8
methyl 1-pyrenylmethyl ether

| Conditions | Yield |
|---|---|
| for 2h; Quantum yield; Irradiation; | A 80% B n/a |

-
-
16738-12-8
methyl α-oxo-1-naphthaleneacetate

-
-
86-87-3
naphth-1-yl acetic acid

| Conditions | Yield |
|---|---|
| Stage #1: methyl α-oxo-1-naphthaleneacetate With hydrazine In propylene glycol at 130℃; for 2h; Stage #2: With sodium hydroxide In propylene glycol at 80℃; for 8h; Solvent; Temperature; Reflux; | 78% |

| Conditions | Yield |
|---|---|
| With N,N,N,N,-tetramethylethylenediamine; α,α′-bis(2-pyridyl(tert-butyl)phosphino)-o-xylene; palladium diacetate In N,N-dimethyl-formamide at 115℃; for 12h; Inert atmosphere; | 75% |

| Conditions | Yield |
|---|---|
| With manganese; dichlorobis(trimethylphosphine)nickel In N,N-dimethyl-formamide at 20℃; under 760.051 Torr; for 48h; Schlenk technique; | 64% |

-
-
86-87-3
naphth-1-yl acetic acid

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate; water In 1,4-dioxane at 175℃; for 0.5h; Microwave irradiation; Green chemistry; | 39% |

| Conditions | Yield |
|---|---|
| With sodium acetate; palladium on activated charcoal In ethanol under 2327.2 Torr; for 1.5h; Ambient temperature; | 24% |
-
-
60-29-7
diethyl ether

-
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

-
-
86-87-3
naphth-1-yl acetic acid


| Conditions | Yield |
|---|---|
| With diethyl ether; magnesium anschliessendes Behandeln mit Chloressigsaeure; |
-
-
2489-86-3
1-allylnaphthalene

-
A
-
86-87-3
naphth-1-yl acetic acid

-
B
-
120727-51-7
3-[1]naphthyl-propane-1,2-diol

| Conditions | Yield |
|---|---|
| With potassium permanganate; ethanol; water weiteres Reagens: Natriumacetat; |
-
-
84300-72-1
4-(naphthalen-1-yl-thioacetyl)-morpholine

-
-
86-87-3
naphth-1-yl acetic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; acetic acid | |
| With potassium hydroxide |
-
-
57427-83-5, 57427-99-3, 58130-84-0
4-naphthalen-1-ylmethylene-2-phenyl-4H-oxazol-5-one

-
-
86-87-3
naphth-1-yl acetic acid

| Conditions | Yield |
|---|---|
| nachfolgend Oxydation mit Wasserstoffperoxyd; |
-
-
26153-26-4
1-naphthylglyoxylic acid

-
-
86-87-3
naphth-1-yl acetic acid

| Conditions | Yield |
|---|---|
| With phosphorus; hydrogen iodide at 160℃; | |
| With potassium hydroxide; hydrazine hydrate |

| Conditions | Yield |
|---|---|
| With thionyl chloride | 100% |
| With thionyl chloride for 2h; Reflux; | 100% |
| With thionyl chloride; N,N-dimethyl-formamide for 4h; | 100% |

| Conditions | Yield |
|---|---|
| With thionyl chloride for 3.5h; Heating; | 100% |
| With iron(III) sulfate; sulfuric acid for 3.5h; Heating; | 86% |
| With sulfuric acid |

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 1h; Heating; | 100% |
| With thionyl chloride at 20℃; | 99% |
| With iron(III) sulfate; sulfuric acid for 1.5h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; copper diacetate In acetic acid for 2h; Heating; | 100% |
-
-
86-87-3
naphth-1-yl acetic acid

-
-
75-03-6
ethyl iodide

-
-
74327-46-1, 74327-47-2, 74364-83-3, 15410-62-5
2-(naphthalen-1-yl)butanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: naphth-1-yl acetic acid With N,N,N,N,N,N-hexamethylphosphoric triamide; lithium diisopropyl amide In tetrahydrofuran; n-heptane; ethylbenzene at 20℃; for 0.5h; Stage #2: ethyl iodide In tetrahydrofuran; n-heptane; ethylbenzene at 20℃; for 0.0833333h; Further stages.; | 100% |
| Stage #1: naphth-1-yl acetic acid With n-butyllithium In tetrahydrofuran; hexane at 0℃; for 1h; Stage #2: ethyl iodide at 0 - 20℃; | |
| Stage #1: naphth-1-yl acetic acid With n-butyllithium In tetrahydrofuran; hexanes at 0℃; for 0.166667h; Inert atmosphere; Stage #2: ethyl iodide In tetrahydrofuran; hexanes Inert atmosphere; | |
| Stage #1: naphth-1-yl acetic acid With n-butyllithium In tetrahydrofuran; hexane at -78 - 0℃; for 2.16667h; Inert atmosphere; Stage #2: ethyl iodide In tetrahydrofuran; hexane at 0 - 20℃; Inert atmosphere; |
-
-
86-87-3
naphth-1-yl acetic acid

-
-
21715-90-2
endo-N-hydroxy-5-norbornene-2,3-dicarboxyimide

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide | 99% |
-
-
2033-24-1
cycl-isopropylidene malonate

-
-
86-87-3
naphth-1-yl acetic acid

-
-
288270-18-8
5-[1-hydroxy-2-(nalphthalen-1-yl)ethylidene]-2,2-dimethyl[1,3]dioxane-4,6-dione

| Conditions | Yield |
|---|---|
| Stage #1: cycl-isopropylidene malonate; naphth-1-yl acetic acid With dmap In dichloromethane at -10℃; for 0.75h; Stage #2: With dicyclohexyl-carbodiimide In dichloromethane at -10 - 20℃; | 99% |
| Stage #1: naphth-1-yl acetic acid With 1,1'-carbonyldiimidazole In dichloromethane for 0.5h; Stage #2: cycl-isopropylidene malonate In dichloromethane for 12h; | |
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; Inert atmosphere; | |
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; | |
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In acetonitrile at 60℃; for 4h; | 99% |
-
-
86-87-3
naphth-1-yl acetic acid

-
-
100-46-9
benzylamine

-
-
158833-27-3
N-benzyl-2-(naphthalen-1-yl)acetamide

| Conditions | Yield |
|---|---|
| With borane-ammonia complex In 5,5-dimethyl-1,3-cyclohexadiene for 6h; Reflux; | 99% |
| Stage #1: naphth-1-yl acetic acid With 1,3,5-trichloro-2,4,6-triazine; caesium carbonate In dichloromethane at 20℃; for 0.333333h; Inert atmosphere; Sonication; Stage #2: benzylamine In dichloromethane Sonication; Inert atmosphere; | 89% |
| Stage #1: naphth-1-yl acetic acid With titanium(IV) isopropylate In tetrahydrofuran at 40 - 70℃; Molecular sieve; Inert atmosphere; Stage #2: benzylamine In tetrahydrofuran at 70℃; Molecular sieve; Inert atmosphere; | 79% |
| Stage #1: naphth-1-yl acetic acid With 4-(dimethylamino)pyridine N-oxide; phenylboronic acid In benzene at 20℃; for 0.0833333h; Molecular sieve; Reflux; Stage #2: benzylamine In fluorobenzene for 3h; Reflux; Molecular sieve; | 20% |
| Stage #1: naphth-1-yl acetic acid With 4-methyl-morpholine; isobutyl chloroformate In tetrahydrofuran; N,N-dimethyl-formamide at -15℃; Inert atmosphere; Stage #2: benzylamine In tetrahydrofuran; N,N-dimethyl-formamide at -15 - 20℃; for 1.5h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| Stage #1: naphth-1-yl acetic acid With 1,3,5-trichloro-2,4,6-triazine; potassium carbonate In tetrahydrofuran for 0.0166667h; Milling; Stage #2: With ammonium thiocyanate In tetrahydrofuran for 0.0833333h; Milling; | 98% |
| Stage #1: naphth-1-yl acetic acid With thionyl chloride In dichloromethane at 0℃; for 2h; Stage #2: With ammonium hydroxide In tetrahydrofuran at 0 - 20℃; | 88% |
| With phosphorus pentachloride Behandeln mit Ammoniumcarbonat; |

| Conditions | Yield |
|---|---|
| In toluene for 12h; Reflux; | 98% |
| In toluene at 110℃; for 24h; | 92% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In acetonitrile at 60℃; for 4h; | 98% |
-
-
86-87-3
naphth-1-yl acetic acid

-
-
1038502-72-5
1-tosyl-4-vinyl-1,4-dihydro-2H-benzo[d] [1,3]oxazin-2-one

| Conditions | Yield |
|---|---|
| Stage #1: naphth-1-yl acetic acid With pivaloyl chloride; caesium carbonate In 1,3,5-trimethyl-benzene at 0℃; for 0.5h; Inert atmosphere; Stage #2: 1-tosyl-4-vinyl-1,4-dihydro-2H-benzo[d] [1,3]oxazin-2-one With tris-(dibenzylideneacetone)dipalladium(0); (R)-BIDIME In 1,3,5-trimethyl-benzene at 0℃; Inert atmosphere; Molecular sieve; stereoselective reaction; | 98% |
-
-
86-87-3
naphth-1-yl acetic acid


| Conditions | Yield |
|---|---|
| Stage #1: naphth-1-yl acetic acid; (2S,4S)-4-hydroxy-4-((3aR,7S,8aS)-7-methyl-3-methylene-2-oxo-3,3a,4,7,8,8a-hexahydro-2H-cyclohepta[b]furan-6-yl)butan-2-yl acetate With dmap In dichloromethane at 0℃; Stage #2: With dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 3.08333h; | 97% |
-
-
86-87-3
naphth-1-yl acetic acid

-
-
74-88-4
methyl iodide

-
-
19950-47-1, 22561-77-9, 24252-61-7, 3117-51-9
2-(naphthalen-1-yl)propanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: naphth-1-yl acetic acid With lithium diisopropyl amide In tetrahydrofuran at -78 - 0℃; Stage #2: methyl iodide In tetrahydrofuran at 20℃; Further stages.; | 96% |
| Stage #1: naphth-1-yl acetic acid With lithium diisopropyl amide In tetrahydrofuran at -78 - 0℃; for 1.41667h; Inert atmosphere; Stage #2: methyl iodide In tetrahydrofuran at 20℃; Time; | 96% |
| Stage #1: naphth-1-yl acetic acid With N,N,N,N,N,N-hexamethylphosphoric triamide; lithium diisopropyl amide In tetrahydrofuran; n-heptane; ethylbenzene at 20℃; for 0.5h; Stage #2: methyl iodide In tetrahydrofuran; n-heptane; ethylbenzene at 20℃; for 0.0833333h; Further stages.; | 86% |
-
-
86-87-3
naphth-1-yl acetic acid

-
-
7677-24-9
trimethylsilyl cyanide

-
-
70067-70-8
3-(naphthalen-1-yl)propanenitrile

| Conditions | Yield |
|---|---|
| Stage #1: naphth-1-yl acetic acid With indium(III) bromide; 1,1,3,3-Tetramethyldisiloxane; iodine In chloroform at 60℃; Inert atmosphere; Stage #2: trimethylsilyl cyanide With tetrabutyl ammonium fluoride In tetrahydrofuran; chloroform at 60℃; for 5h; Reagent/catalyst; Inert atmosphere; chemoselective reaction; | 96% |

| Conditions | Yield |
|---|---|
| With oxygen; copper(II) trifluoroacetate; urea In dimethyl sulfoxide at 120℃; for 22h; Green chemistry; | 96% |
| With 1,10-Phenanthroline; oxygen; copper(II) oxide; potassium ferrocyanide In dimethyl sulfoxide at 120℃; under 11251.1 Torr; for 40h; Autoclave; | 70% |
| With iron(III) trifluoromethanesulfonate; sodium nitrite In dimethyl sulfoxide at 50℃; for 10h; Inert atmosphere; Sealed tube; | 69% |
-
-
86-87-3
naphth-1-yl acetic acid

-
-
138585-08-7
(4-((tert-butyldimethylsilyl)oxy)phenyl)methanol

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 2.5h; | 96% |

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In 1,4-dioxane at 20℃; for 2h; Inert atmosphere; | 95% |
-
-
442640-22-4
3-descladinosyl-9-deokso-9a-aza-9a-(3-aminopropyl)-9a-homoeritromicin A

-
-
86-87-3
naphth-1-yl acetic acid

| Conditions | Yield |
|---|---|
| Stage #1: naphth-1-yl acetic acid With PS-carbodiimide In dichloromethane at 20℃; for 1h; Stage #2: 3-descladinosyl-9-deokso-9a-aza-9a-(3-aminopropyl)-9a-homoeritromicin A In dichloromethane at 20℃; for 2h; | 95% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; dicyclohexyl-carbodiimide In tetrahydrofuran at 20℃; | 95% |
-
-
86-87-3
naphth-1-yl acetic acid

-
-
119-36-8
methyl salicylate

-
A
-
2876-78-0
methyl 1-naphthylacetate

-
B
-
69-72-7
salicylic acid

| Conditions | Yield |
|---|---|
| Stage #1: naphth-1-yl acetic acid With potassium carbonate In N,N-dimethyl acetamide at 110℃; for 0.5h; Stage #2: methyl salicylate at 110℃; for 24h; | A 95% B n/a |


| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 95% |

| Conditions | Yield |
|---|---|
| With tricarbonyl(η(4)-cycloocta-1,5-diene)iron; phenylsilane In tetrahydrofuran at 20℃; for 24h; Inert atmosphere; UV-irradiation; chemoselective reaction; | 94% |
| With borane In tetrahydrofuran for 1h; Ambient temperature; | 90% |
| With tin(II) trifluoromethanesulfonate; tris(2,4-pentanedionato)ruthenium(III); hydrogen; [2-((diphenylphospino)methyl)-2-methyl-1,3-propanediyl]bis[diphenylphosphine] In water; toluene at 160℃; under 45004.5 Torr; for 48h; Autoclave; | 87% |
-
-
86-87-3
naphth-1-yl acetic acid


| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 10h; | 94% |
1-Naphthalene acetic acid Consensus Reports
Reported in EPA TSCA Inventory.
1-Naphthalene acetic acid Specification
The 1-Naphthalene acetic acid, with the CAS registry number 86-87-3, is also known as Naphthalene-1-acetic acid. It belongs to the product categories of Naphthalene derivatives; Organics; Organic acids; Auxins; Biochemistry; Plant Growth Regulators; Plant Hormones. Its EINECS number is 201-705-8. This chemical's molecular formula is C12H10O2 and molecular weight is 186.21. What's more, its systematic name is 1-Naphthylacetic acid. Its classification codes are: (1)Agricultural Chemical; (2)Growth regulator / Fertilizer; (3)Herbicide; (4)Mutation data; (5)Skin / Eye Irritant. This chemical should be sealed and stored in a cool and dry place. Moreover, it should be protected from fire and moisture. This chemical is a synthetic plant hormone in the auxin family and is an ingredient in many commercial plant rooting horticultural products. It is a rooting agent and used for the vegetative propagation of plants from stem and leaf cutting. It is also used for plant tissue culture.
Physical properties of 1-Naphthalene acetic acid are: (1)ACD/LogP: 2.529; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.30; (4)ACD/LogD (pH 7.4): -0.49; (5)ACD/BCF (pH 5.5): 2.91; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 33.41; (8)ACD/KOC (pH 7.4): 1.00; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 37.3 Å2; (13)Index of Refraction: 1.652; (14)Molar Refractivity: 55.209 cm3; (15)Molar Volume: 150.992 cm3; (16)Polarizability: 21.887×10-24cm3; (17)Surface Tension: 54.1 dyne/cm; (18)Density: 1.233 g/cm3; (19)Flash Point: 270.097 °C; (20)Enthalpy of Vaporization: 65.454 kJ/mol; (21)Boiling Point: 373.207 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
Preparation: this chemical can be prepared by naphthalen-1-yl-acetonitrile at the temperature of 30 °C. This reaction will need reagent potassium phosphate buffer with the reaction time of 96 hours. The yield is about 91.3%.
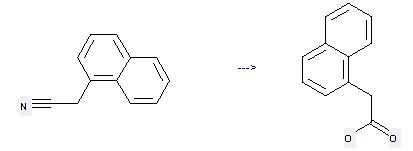
Uses of 1-Naphthalene acetic acid: it can be used to produce 2-[1]naphthyl-3-phenyl-acrylic acid at the temperature of 120 °C. It will need reagents Ac2O, Et3N with the reaction time of 24 hours. The yield is about 90%.
![1-Naphthalene acetic acid can be used to produce 2-[1]naphthyl-3-phenyl-acrylic acid at the temperature of 120 °C](/UserFilesUpload/Uses of 1-Naphthalene acetic acid.jpeg)
When you are using this chemical, please be cautious about it as the following:
This chemical is harmful if swallowed. It is irritating to eyes, respiratory system and skin. This substance can cause burns. It has a risk of serious damage to eyes and a possible risk of irreversible effects. In case of contact with eyes, you should rinse immediately with plenty of water and seek medicl advice. When using it, you need wear suitable protective clothing, gloves and eye/face protection and you must avoid contact with skin and eyes. You should not breathe dust. After using it, you must take off immediately all contaminated clothing. In case of accident or if you feel unwell, you should seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(O)Cc2cccc1ccccc12
(2)Std. InChI: InChI=1S/C12H10O2/c13-12(14)8-10-6-3-5-9-4-1-2-7-11(9)10/h1-7H,8H2,(H,13,14)
(3)Std. InChIKey: PRPINYUDVPFIRX-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 609mg/kg (609mg/kg) | Toho Igakkai Zasshi. Journal of Medical Society of Toho University. Vol. 15, Pg. 443, 1968. | |
| mouse | LD50 | oral | 743mg/kg (743mg/kg) | Toho Igakkai Zasshi. Journal of Medical Society of Toho University. Vol. 14, Pg. 132, 1967. | |
| mouse | LD50 | subcutaneous | 733mg/kg (733mg/kg) | Toho Igakkai Zasshi. Journal of Medical Society of Toho University. Vol. 15, Pg. 443, 1968. | |
| rabbit | LD50 | skin | > 5gm/kg (5000mg/kg) | Pesticide Manual. Vol. 9, Pg. 607, 1991. | |
| rat | LC50 | inhalation | > 207gm/m3 (207000mg/m3) | Pesticide & Toxic Chemical News. Vol. 9, Pg. 10, 1980. | |
| rat | LD50 | intraperitoneal | 100mg/kg (100mg/kg) | Pesticide & Toxic Chemical News. Vol. 9, Pg. 10, 1980. | |
| rat | LD50 | oral | 1gm/kg (1000mg/kg) | "Wirksubstanzen der Pflanzenschutz und Schadlingsbekampfungsmittel," Perkow, W., Berlin, Verlag Paul Parey, 1971-1976Vol. -, Pg. -, 1971/1976. |
Related Products
- 1-Naphthalene acetic acid
- 1-Naphthaleneacetic acid, alpha-(2-(dimethylamino)ethyl)-alpha-methyl-, hydrochloride
- 1-Naphthaleneaceticacid, butyl ester
- 1-Naphthaleneaceticacid, decahydro-
- 1-Naphthaleneaceticacid, hydrazide
- 1-Naphthaleneaceticacid, potassium salt (1:1)
- 1-Naphthaleneacetylchloride
- 1-Naphthaleneboronic acid
- 1-Naphthalenebutanoicacid, 1,2,3,4-tetrahydro-
- 1-Naphthalenebutanoicacid, g-oxo-, ethyl ester
- 86873-25-8
- 86873-60-1
- 868736-42-9
- 868755-42-4
- 868755-44-6
- 868755-46-8
- 868755-47-9
- 868755-48-0
- 868755-49-1
- 868755-50-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View