-
Name
2,2,6,6-Tetramethylpiperidine
- EINECS 212-199-3
- CAS No. 768-66-1
- Article Data78
- CAS DataBase
- Density 0.787 g/cm3
- Solubility miscible with water
- Melting Point -59 °C
- Formula C9H19N
- Boiling Point 157.6 °C at 760 mmHg
- Molecular Weight 141.257
- Flash Point 24.4 °C
- Transport Information UN 1992 3/PG 3
- Appearance clear colorless to light yellow-green liquid
- Safety 26-37/39-16
- Risk Codes 10-22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  F,
F,  C
C
- Synonyms Norpempidine;2,2,6,6-Tetramethylpiperidien;
- PSA 12.03000
- LogP 2.64590
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen; platinum(IV) oxide under 1.5 Torr; for 40h; | 100% |
| With potassium hydroxide; hydrazine hydrate In diethylene glycol at 210℃; Wolff-Kishner-Huang reduction; | 83% |
| With potassium hydroxide; hydrazine In water; diethylene glycol at 127 - 200℃; Wolff-Kishner reduction; | 82% |
-
-
2564-83-2, 45842-10-2
2,2,6,6-Tetramethyl-1-piperidinyloxy free radical


-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
B
-
119-61-9
benzophenone

-
C
-
102261-92-7
1-benzyloxy-2,2,6,6-tetramethylpiperidine

| Conditions | Yield |
|---|---|
| In toluene at 100℃; for 8h; Rate constant; Thermodynamic data; ΔH(excit.), ΔS(excit.); | A 90% B 95% C 92% |

-
-
2564-83-2, 45842-10-2
2,2,6,6-Tetramethyl-1-piperidinyloxy free radical

-
-
622-47-9
4-tolylacetic acid

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

| Conditions | Yield |
|---|---|
| Stage #1: 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; 4-tolylacetic acid With N4,N4,N7,N7-tetramethyl-1,10-phenanthroline-4,7-diamine; iron(II) acetate In tetrahydrofuran at 60℃; for 96h; Molecular sieve; Inert atmosphere; Stage #2: With triethylamine In acetone Kinetics; Reagent/catalyst; Solvent; | A n/a B 90% |


-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

| Conditions | Yield |
|---|---|
| In sulfuric acid; water | 89.6% |

-
-
145178-53-6
2,3-bis(trimethylsilyl)-1,3-butadiene-1,4-dione

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
B
-
102261-92-7
1-benzyloxy-2,2,6,6-tetramethylpiperidine

| Conditions | Yield |
|---|---|
| With 2,2,6,6-tetramethyl-piperidine-N-oxyl In toluene at 90℃; for 3h; | A 85% B 76% C 80% |


-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

| Conditions | Yield |
|---|---|
| With 4-methoxy-9H-thioxanthen-9-one; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In dichloromethane; acetone at 20℃; for 48h; Reagent/catalyst; Solvent; Sealed tube; Irradiation; Inert atmosphere; | A n/a B 84% |
-
-
2564-83-2, 45842-10-2
2,2,6,6-Tetramethyl-1-piperidinyloxy free radical

-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

| Conditions | Yield |
|---|---|
| With tert-Butyl peroxybenzoate; 1-cyano-1,2-benziodoxol-3-(1H)-one at 110℃; for 12h; Inert atmosphere; Glovebox; | 76% |
| With thiophosgene at 20℃; for 24h; | 37% |
| Multi-step reaction with 2 steps 1: 1) FClO3, BF3*(C2H5)2O / 1) hexane, 0 deg C 2: 1) FClO3, BF3*(C2H5)2O / 1) hexane, 0 deg C View Scheme |
-
-
108-88-3
toluene

-
-
618453-08-0
N-triphenylacetoxy-2,2,6,6-tetramethylpiperidine

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
B
-
2294-94-2
1,1,1,2-tetraphenylethane

| Conditions | Yield |
|---|---|
| at 146℃; for 40h; | A 78 % Chromat. B 74% |

-
-
2564-83-2
2,2,6,6-tetramethyl-piperidine-N-oxyl

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
B
-
119-61-9
benzophenone

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 0.0833333h; Inert atmosphere; Schlenk technique; | A n/a B n/a C 72% |

-
-
366818-95-3
2-(2,2,6,6-tetramethyl-piperidin-1-yloxy)-hept-6-enoic acid 2,2,6,6-tetramethyl-piperidin-1-yl ester

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

| Conditions | Yield |
|---|---|
| In tert-butyl alcohol at 130℃; for 24h; | A 8% B 70% |
-
-
826-36-8
2,2,6,6-Tetramethyl-4-piperidone

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
B
-
2403-88-5
4-hydroxy-2,2,6,6-tetramethylpiperidine

| Conditions | Yield |
|---|---|
| With sulfuric acid at 25 - 30℃; for 4h; diapraghm electrolyzer, Cd cathode, concentration of II 0.65 mol/l; | A 66.1% B 20% |
| With sulfuric acid at 25 - 30℃; for 4h; diapraghm electrolyzer, Cd cathode, concentration of II 1.3 mol/l; | A 27.7% B 54.6% |
| With sulfuric acid at 24.85 - 29.85℃; Product distribution; Mechanism; electrochemical reduction (Cd or Pb cathodes, i=100 mA/cn2); var. reagents and temp.; |
-
-
7031-93-8
2,2,6,6-tetramethylpiperidin-1-ol

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
B
-
2564-83-2, 45842-10-2
2,2,6,6-Tetramethyl-1-piperidinyloxy free radical

| Conditions | Yield |
|---|---|
| In diethyl ether; acetonitrile at 20℃; for 8h; Inert atmosphere; Schlenk technique; Sealed tube; | A n/a B 64% |
| at 70℃; for 2h; Schlenk technique; Inert atmosphere; | A 32 %Chromat. B 67 %Chromat. |
-
-
104172-64-7
chloro(diphenylmethyl)(2,2,6,6-tetramethylpiperidino)borane

-
-
38227-87-1
2,2,6,6-tetramethylpiperidinyl-lithium

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
B
-
105309-74-8
benzyl(diphenylmethyl)(2,2,6,6-tetramethylpiperidino)borane

| Conditions | Yield |
|---|---|
| In toluene byproducts: LiCl; addn. of the lithiopiperidine in toluene to the borane in toluene and refluxing for 3 h; filtn. (LiCl), concn. (high vac.), distn. (vac.) and recrystn. from ether; elem. anal.; | A n/a B 63% |


| Conditions | Yield |
|---|---|
| Stage #1: [Li(μ-2,2,6,6-tetramethylpiperidide)(μ-bis[2-(N,N-dimethylamino)ethyl]ether)Al(isobutyl)2] With zinc diacetate In tetrahydrofuran Inert atmosphere; Stage #2: para-iodoanisole With dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; palladium diacetate In tetrahydrofuran Inert atmosphere; | A n/a B 62% |

-
-
2564-83-2, 45842-10-2
2,2,6,6-Tetramethyl-1-piperidinyloxy free radical

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
B
-
7605-48-3
bis(4-tert-butylphenyl)disulfide

| Conditions | Yield |
|---|---|
| With 4-t-butylbenzenethiol In benzene for 0.5h; Ambient temperature; Further byproducts given; | A 10% B 10% C 9% D 56% |

-
-
2564-83-2, 45842-10-2
2,2,6,6-Tetramethyl-1-piperidinyloxy free radical

-
-
2396-68-1
4-t-butylbenzenethiol

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
B
-
7605-48-3
bis(4-tert-butylphenyl)disulfide

| Conditions | Yield |
|---|---|
| In benzene for 0.5h; Ambient temperature; Further byproducts given; | A 10% B 10% C 9% D 56% |

-
-
2396-68-1
4-t-butylbenzenethiol

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
B
-
7605-48-3
bis(4-tert-butylphenyl)disulfide

| Conditions | Yield |
|---|---|
| With 2,2,6,6-tetramethyl-piperidine-N-oxyl In benzene for 0.5h; Ambient temperature; Further byproducts given; | A 10% B 10% C 9% D 56% |

-
-
2564-83-2, 45842-10-2
2,2,6,6-Tetramethyl-1-piperidinyloxy free radical

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
B
-
7031-93-8
2,2,6,6-tetramethylpiperidin-1-ol

| Conditions | Yield |
|---|---|
| With tris-(trimethylsilyl)silane In benzene at 80℃; for 3h; Product distribution; Mechanism; other reagents, reaction time, solvent, effect of irradiation and addition of initiators; | A 56% B 41% |
-
-
79-55-0
1,2,2,6,6-pentamethylpiperidine

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
B
-
54262-97-4
2,2,6,6-tetramethylpiperidine-1-carboxaldehyde

| Conditions | Yield |
|---|---|
| With ruthenium tetroxide In tetrachloromethane for 2h; | A 4% B 53% |
| With 1,4-dimethylnaphtalene-1,4-endoperoxide In acetonitrile at 40℃; for 4h; Product distribution; Kinetics; | A 23 % Chromat. B 1.2 % Chromat. |
-
-
2564-83-2, 45842-10-2
2,2,6,6-Tetramethyl-1-piperidinyloxy free radical

-
-
108-98-5
thiophenol

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
B
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| In benzene for 0.5h; Ambient temperature; Further byproducts given; | A 9% B 8% C 14% D 49% |

-
-
2564-83-2, 45842-10-2
2,2,6,6-Tetramethyl-1-piperidinyloxy free radical

-
-
108-98-5
thiophenol

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

| Conditions | Yield |
|---|---|
| In benzene for 0.5h; Ambient temperature; Further byproducts given; | A 9% B 5% C 14% D 49% |

-
-
2564-83-2, 45842-10-2
2,2,6,6-Tetramethyl-1-piperidinyloxy free radical

-
-
108-98-5
thiophenol

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

| Conditions | Yield |
|---|---|
| In benzene for 0.5h; Ambient temperature; Further byproducts given; | A 9% B 7% C 14% D 49% |


| Conditions | Yield |
|---|---|
| With 2,2,6,6-tetramethyl-piperidine-N-oxyl In benzene for 0.5h; Ambient temperature; Further byproducts given; | A 9% B 8% C 14% D 49% |

-
-
2564-83-2, 45842-10-2
2,2,6,6-Tetramethyl-1-piperidinyloxy free radical

-
-
106-45-6
para-thiocresol

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
B
-
103-19-5
di(p-tolyl) disulfide

-
C
-
30680-88-7
2,2,6,6-tetarmethylpiperidinium p-toluenesulphonate

| Conditions | Yield |
|---|---|
| In benzene for 0.5h; Ambient temperature; Further byproducts given; | A 7% B 12% C 47% D 21% |

-
-
106-45-6
para-thiocresol

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
B
-
103-19-5
di(p-tolyl) disulfide

-
C
-
30680-88-7
2,2,6,6-tetarmethylpiperidinium p-toluenesulphonate

| Conditions | Yield |
|---|---|
| With 2,2,6,6-tetramethyl-piperidine-N-oxyl In benzene for 0.5h; Ambient temperature; Further byproducts given; | A 7% B 12% C 47% D 21% |

-
-
2564-83-2, 45842-10-2
2,2,6,6-Tetramethyl-1-piperidinyloxy free radical

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
B
-
131309-38-1
1-Ethanesulfonyl-2,2,6,6-tetramethyl-piperidine

| Conditions | Yield |
|---|---|
| With ethanethiol In benzene Yields of byproduct given; | A 45% B n/a |
-
-
79-55-0
1,2,2,6,6-pentamethylpiperidine

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
B
-
5132-07-0
TEMPO

-
C
-
54262-97-4
2,2,6,6-tetramethylpiperidine-1-carboxaldehyde

| Conditions | Yield |
|---|---|
| With sodium periodate; ruthenium tetroxide In tetrachloromethane; water for 5h; | A 4% B 7% C 41% |

-
-
330938-05-1
phenylacetic acid 2,2,6,6-tetramethylpiperidin-1-yl ester

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
B
-
103-82-2
phenylacetic acid

| Conditions | Yield |
|---|---|
| In tert-butyl alcohol at 130℃; for 168h; | A 30% B 35% |
-
-
2564-83-2, 45842-10-2
2,2,6,6-Tetramethyl-1-piperidinyloxy free radical

-
-
100-46-9
benzylamine

-
A
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
B
-
7031-93-8
2,2,6,6-tetramethylpiperidin-1-ol

-
C
-
74601-40-4
phenyl(2,2,6,6-tetramethylpiperidin-1-yl)methanone

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; zinc dibromide In pyridine; water at 80℃; for 16h; | A n/a B n/a C 35% |


| Conditions | Yield |
|---|---|
| With Hg(II)-EDTA | 28% |
| With Hg(II)-EDTA Product distribution; other reagent: Hg(OAc)2; also in HCOOH as solvent; | 28% |
-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
-
14775-42-9
2,2,5,5-tetramethyl-4-(toluene-4-sulfonyloxy)-hexan-3-one

-
-
73333-60-5
2-(2,3-Di-tert-butyl-oxiranyl)-4-methyl-benzenesulfonic acid; compound with 2,2,6,6-tetramethyl-piperidine

| Conditions | Yield |
|---|---|
| With methyllithium Mechanism; | 100% |
| With methyllithium Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With Oxone; silica gel In water at 80℃; for 24h; Oxidation; | 100% |

| Conditions | Yield |
|---|---|
| With C36H47Cl2N2P2PdTi(2+) In chloroform-d1 at 20℃; for 0.0833333h; Glovebox; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,2,6,6-tetramethyl-piperidine; benzylmagnesium chloride In tetrahydrofuran at 40℃; Inert atmosphere; Schlenk technique; Stage #2: zinc trimethylacetate In tetrahydrofuran at 20℃; for 0.333333h; Inert atmosphere; Schlenk technique; | 100% |
| Stage #1: 2,2,6,6-tetramethyl-piperidine; benzylmagnesium chloride In tetrahydrofuran at 20 - 40℃; for 12h; Inert atmosphere; Schlenk technique; Stage #2: zinc trimethylacetate In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Schlenk technique; |

-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
-
32579-76-3
1-chloro-2,2,6,6-tetramethylpiperidine

| Conditions | Yield |
|---|---|
| With chlorine; sodium hydroxide In water at 5 - 20℃; for 1.5h; Reagent/catalyst; Time; Inert atmosphere; | 99% |
| With sodium hypochlorite pentahydrate; sodium acetate trihydrate; acetic acid In water at 20℃; for 2h; | 91% |
| With hydrogenchloride; sodium chloride In tetrachloromethane at 20℃; electrolysis; | 72% |

| Conditions | Yield |
|---|---|
| With sodium In chlorobenzene; toluene | 99% |
-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
-
124-38-9
carbon dioxide

-
-
79-55-0
1,2,2,6,6-pentamethylpiperidine

| Conditions | Yield |
|---|---|
| With phenylsilane In N,N-dimethyl acetamide at 60℃; for 2h; Sealed tube; | 99% |
| With phenylsilane In N,N-dimethyl-formamide at 90℃; under 760.051 Torr; for 24h; Schlenk technique; | 95% |
| With diphenylsilane; C21H41N3NiP2 In acetonitrile at 120℃; under 2052.14 Torr; for 24h; Autoclave; | 84% |
-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
-
76-05-1
trifluoroacetic acid

-
-
563-47-3
3-Chloro-2-methylpropene

| Conditions | Yield |
|---|---|
| Stage #1: 2,2,6,6-tetramethyl-piperidine; 3-Chloro-2-methylpropene With C36H45Cl2N2P2PdTi(1+) In dichloromethane at 20℃; for 0.166667h; Stage #2: trifluoroacetic acid In methanol; dichloromethane Reagent/catalyst; | 99% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite at 75 - 100℃; Nitrosation; | 98% |
| With hydrogenchloride; sodium nitrite at 75 - 100℃; Nitrosation; | 98% |
| With hydrogenchloride; sodium nitrite for 96h; Heating; | 89% |
-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
-
104-15-4
toluene-4-sulfonic acid

-
-
30680-88-7
2,2,6,6-tetarmethylpiperidinium p-toluenesulphonate

| Conditions | Yield |
|---|---|
| In acetone at 15 - 20℃; for 0.5h; | 98% |
| In acetonitrile Yield given; |
-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
-
372-09-8
cyanoacetic acid

-
-
1360789-22-5
3-oxo-3-(2,2,6,6-tetramethylpiperidin-1-yl)propanenitrile

| Conditions | Yield |
|---|---|
| Stage #1: cyanoacetic acid With 2-chloro-1-methyl-pyridinium iodide In dichloromethane for 0.25h; Inert atmosphere; Stage #2: 2,2,6,6-tetramethyl-piperidine In dichloromethane at 18℃; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,2,6,6-tetramethyl-piperidine With n-butyllithium In pentane at -78℃; for 1h; Inert atmosphere; Stage #2: zinc(II) chloride In diethyl ether; pentane at -78 - 23℃; Inert atmosphere; | 98% |
| Stage #1: 2,2,6,6-tetramethyl-piperidine With n-butyllithium In hexane at 0℃; for 1h; Inert atmosphere; Schlenk technique; Stage #2: zinc(II) chloride In diethyl ether; hexane at 0℃; for 48h; Inert atmosphere; Schlenk technique; | 58% |
| Stage #1: 2,2,6,6-tetramethyl-piperidine With n-butyllithium In hexane at 0℃; for 1h; Inert atmosphere; Schlenk technique; Stage #2: zinc(II) chloride In diethyl ether for 48h; Inert atmosphere; Schlenk technique; | 58% |
-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
1252594-28-7
2,2,6,6-tetramethylpiperidinium triflate

| Conditions | Yield |
|---|---|
| In ethyl acetate at 15 - 20℃; for 0.5h; | 98% |
-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
-
13450-90-3
Gallium trichloride

| Conditions | Yield |
|---|---|
| With n-butyllithium In diethyl ether; hexane byproducts: LiCl; under inert atmosphere, piperidine in hexane reacted with n-BuLi in hexane, dropwise addn. of GaCl3 in diethyl ether at room temp. with stirring, refluxed for 2 h; filtered, cooled to -78°C; elem. anal.; | 97% |
-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

| Conditions | Yield |
|---|---|
| With isopropyl chloride; magnesium; lithium chloride In tetrahydrofuran; toluene at 40 - 80℃; under 5171.62 Torr; Inert atmosphere; Flow reactor; | 97% |
| With TurboGrignard In tetrahydrofuran at 20℃; for 48h; Inert atmosphere; | |
| With TurboGrignard In tetrahydrofuran for 48h; Inert atmosphere; |
-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
-
13894-21-8
ethynyl p-tolyl sulfone

| Conditions | Yield |
|---|---|
| In dichloromethane for 1h; Ambient temperature; | 96% |
-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
-
922-67-8
propynoic acid methyl ester

-
-
118061-26-0
(E)-1-methoxycarbonyl-2-ethylene

| Conditions | Yield |
|---|---|
| In dichloromethane for 1h; Ambient temperature; | 96% |

| Conditions | Yield |
|---|---|
| In diethyl ether | 96% |
| In diethyl ether |

| Conditions | Yield |
|---|---|
| In acetone at 15 - 20℃; for 0.5h; | 96% |
-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
-
81428-20-8
(1-Brom-2,2-dimethyl-1-phenylpropyl)isocyanat

-
-
81428-34-4
3-(2,2-Dimethyl-1-phenylpropyliden)-1,1-(1,1,5,5-tetramethyl-1,5-pentandiyl)harnstoff

| Conditions | Yield |
|---|---|
| With triethylamine In diethyl ether at -78℃; for 0.25h; | 95% |
-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

| Conditions | Yield |
|---|---|
| With hydrogen fluoride; silica gel | 95% |
-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
-
107819-90-9
1,3-bis(tert-butoxycarbonyl)-2-methyl-2-thiopseudourea

| Conditions | Yield |
|---|---|
| With potassium carbonate; copper(l) chloride In tetrahydrofuran at 40℃; for 12h; | 95% |
-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
-
13450-90-3
Gallium trichloride

- tetramethylpiperidine gallium trichloride
-
154626-03-6
tetramethylpiperidine gallium trichloride

| Conditions | Yield |
|---|---|
| In benzene addn. of tetramethylpiperidine to a stirred soln. of GaCl3 at -78°C, warmed up to 25°C over 2 h, stirring for 6 h; removal of the solvent in vac., extn. in toluene, filtn., concg. of the filtrate, cooling at -30°C for 2 wk, elem. anal.; | 95% |

| Conditions | Yield |
|---|---|
| With hydrogen In toluene at 20℃; under 760.051 Torr; for 1h; | 95% |
| With hydrogen at -80℃; | |
| With hydrogen under 5168.02 Torr; Thermodynamic data; Inert atmosphere; |
-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

| Conditions | Yield |
|---|---|
| With TurboGrignard In tetrahydrofuran at 25℃; for 48h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,2,6,6-tetramethyl-piperidine With n-butyllithium In hexane at -40 - -10℃; for 1h; Stage #2: zinc(II) chloride In tetrahydrofuran; hexane at -10 - 25℃; for 1h; | 95% |
| Stage #1: 2,2,6,6-tetramethyl-piperidine With n-butyllithium In tetrahydrofuran; hexane at -40 - 25℃; for 2h; Inert atmosphere; Stage #2: zinc(II) chloride In tetrahydrofuran; hexane Inert atmosphere; |
-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
-
27286-84-6
((4,5-dimethyl-1,2-phenylene)bis(ethyne-2,1-diyl))dibenzene

-
-
1109-15-5
tris(pentafluorophenyl)borate

| Conditions | Yield |
|---|---|
| In pentane at 20℃; | 95% |

-
-
768-66-1
2,2,6,6-tetramethyl-piperidine

-
-
124-38-9
carbon dioxide

-
-
844504-52-5
tris(pentafluorophenyl)silyl triflate

-
A
-
1252594-28-7
2,2,6,6-tetramethylpiperidinium triflate

| Conditions | Yield |
|---|---|
| In dichloromethane-d2 Glovebox; Schlenk technique; | A n/a B 95% |

2,2,6,6-Tetramethylpiperidine Consensus Reports
Reported in EPA TSCA Inventory.
2,2,6,6-Tetramethylpiperidine Specification
2,2,6,6-Tetramethylpiperidine, with CAS number of 768-66-1, can be called piperidine, 2,2,6,6-tetramethyl-; 2,2,6,6-Tetramethylpiperidin; TEMP. It is an organic compound of the amine class and a clear colorless to light yellow-green liquid. 2,2,6,6-Tetramethylpiperidine (CAS NO.768-66-1) is used in chemistry as a hindered base.
Physical properties about 2,2,6,6-Tetramethylpiperidine are: (1)ACD/LogP: 2.445; (2)ACD/LogD (pH 5.5): -0.65; (3)ACD/LogD (pH 7.4): -0.57; (4)ACD/BCF (pH 5.5): 1.00; (5)ACD/BCF (pH 7.4): 1.00; (6)ACD/KOC (pH 5.5): 1.00; (7)ACD/KOC (pH 7.4): 1.00; (8)#H bond acceptors: 1; (9)#H bond donors: 1; (10)Index of Refraction: 1.417; (11)Molar Refractivity: 45.05 cm3; (12)Molar Volume: 179.316 cm3; (13)Polarizability: 17.859 10-24cm3; (14)Surface Tension: 24.5450000762939 dyne/cm; (15)Density: 0.788 g/cm3; (16)Flash Point: 24.444 °C; (17)Enthalpy of Vaporization: 39.429 kJ/mol; (18)Boiling Point: 157.61 °C at 760 mmHg; (19)Vapour Pressure: 2.73200011253357 mmHg at 25°C
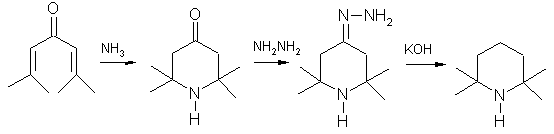
Preparation of 2,2,6,6-Tetramethylpiperidine: The recent production starts with a conjugate addition reaction of ammonia to phorone. The intermediate triacetone amine is then reduced in a Wolff-Kishner reaction.
When you are using this chemical, please be cautious about it as the following:
1. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice;
2. Wear suitable gloves and eye/face protection;
3. Keep away from sources of ignition - No smoking;
You can still convert the following datas into molecular structure:
(1)InChI=1S/C9H19N/c1-8(2)6-5-7-9(3,4)10-8/h10H,5-7H2,1-4H3;
(2)InChIKey=RKMGAJGJIURJSJ-UHFFFAOYSA-N;
(3)SmilesN1C(CCCC1(C)C)(C)C;
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 33mg/kg (33mg/kg) | Nature. Vol. 184, Pg. 1707, 1959. | |
| mouse | LD50 | oral | 220mg/kg (220mg/kg) | Nature. Vol. 184, Pg. 1707, 1959. |
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 7686-77-3
- 7686-78-4
- 76-86-8
- 768-70-7
- 7687-09-4
- 76874-79-8
- 7687-49-2
- 76877-33-3
- 76-87-9
- 76880-29-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View