-
Name
2,3-Dichlorobenzoic acid
- EINECS 200-039-5
- CAS No. 50-45-3
- Article Data30
- CAS DataBase
- Density 1.517 g/cm3
- Solubility slightly soluble in Water
- Melting Point 168-170 °C(lit.)
- Formula C7H4Cl2O2
- Boiling Point 306.9 °C at 760 mmHg
- Molecular Weight 191.014
- Flash Point 139.4 °C
- Transport Information
- Appearance white or kind of white powder or needle form crystallization
- Safety 26-37/39-36-24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Benzoic acid, 2,3-dichloro-;2,3-dichlorobenzoate;2,3-Dichloro benzoic acid;2,3-Dichlorobenzoicacid;
- PSA 37.30000
- LogP 2.69160
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: 1,2-dichloro-benzene With n-butyllithium In tetrahydrofuran at -75℃; for 2h; Stage #2: carbon dioxide In tetrahydrofuran | 88% |
-
-
57915-78-3
1-(bromomethyl)-2,3-dichlorobenzene

-
-
50-45-3
2,3-dichlorbenzoic acid

| Conditions | Yield |
|---|---|
| With sodium periodate; sulfuric acid In water at 95℃; for 12h; | 84% |
-
-
32768-54-0
2,3-dichlorotoluene

-
-
50-45-3
2,3-dichlorbenzoic acid

| Conditions | Yield |
|---|---|
| With sodium periodate; sulfuric acid; lithium bromide In water at 95℃; for 18h; | 75% |
| Stage #1: 2,3-dichlorotoluene With tert.-butylhydroperoxide; sodium hydroxide; tungsten(VI) oxide In water at 80℃; for 10h; Stage #2: With hydrogenchloride In water | 75% |
| With nitric acid at 140℃; im Druckrohr; | |
| With alkaline permanganate |
-
-
6334-18-5
2,3-dichlorobenzylaldehyde

-
A
-
38594-42-2
2,3-dichlorobenzyl alcohol

-
B
-
50-45-3
2,3-dichlorbenzoic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide |

| Conditions | Yield |
|---|---|
| With silver(l) oxide | |
| With potassium permanganate In water; acetonitrile | |
| Stage #1: 2,3-dichlorobenzylaldehyde With ammonia at -33℃; for 1h; Inert atmosphere; Stage #2: With potassium permanganate for 1h; Inert atmosphere; Reflux; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium chlorate; water | |
| With calcium chloride |
-
-
124-38-9
carbon dioxide

-
-
87-61-6
1,2,3-trichlorobenzene

-
A
-
50-45-3
2,3-dichlorbenzoic acid

-
B
-
535-80-8
3-chlorobenzoate

-
C
-
118-91-2
ortho-chlorobenzoic acid

-
D
-
50-30-6
2,6-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide In N,N-dimethyl-formamide at 5℃; electrolysis (I=0.4 A); Yield given. Further byproducts given. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| (i) NaNO2, aq. HCl, (ii) aq. CuSO4, NH2OH*HCl, (iii) FeCl3, aq. HCl; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With sodium peroxide In water at 20℃; for 1h; Oxidation; |
-
-
65-85-0
benzoic acid

-
A
-
50-45-3
2,3-dichlorbenzoic acid

-
B
-
51-44-5
3,4-dichlorbenzoic acid

-
C
-
535-80-8
3-chlorobenzoate

-
-
65-85-0
benzoic acid

-
A
-
50-82-8
2,4,5-trichlorobenzoic acid

-
B
-
50-45-3
2,3-dichlorbenzoic acid

-
C
-
51-44-5
3,4-dichlorbenzoic acid

-
D
-
535-80-8
3-chlorobenzoate

-
-
7647-01-0
hydrogenchloride

-
-
65-85-0
benzoic acid

-
A
-
50-45-3
2,3-dichlorbenzoic acid

-
B
-
535-80-8
3-chlorobenzoate

-
C
-
118-91-2
ortho-chlorobenzoic acid

-
-
98-08-8
α,α,α-trifluorotoluene

-
-
7647-18-9
antimonypentachloride

-
A
-
50-45-3
2,3-dichlorbenzoic acid

-
B
-
74-11-3
para-chlorobenzoic acid

-
C
-
535-80-8
3-chlorobenzoate

| Conditions | Yield |
|---|---|
| Chlorierung; anschliessende Verseifung; |

-
-
124-38-9
carbon dioxide

-
-
87-61-6
1,2,3-trichlorobenzene

-
A
-
50-75-9
2,3,4-trichlorobenzoic acid

-
B
-
50-45-3
2,3-dichlorbenzoic acid

-
C
-
50-30-6
2,6-dichlorobenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 1,2,3-trichlorobenzene With sec.-butyllithium In tetrahydrofuran; cyclohexane at -75℃; for 0.75h; Stage #2: carbon dioxide In tetrahydrofuran; cyclohexane Title compound not separated from byproducts; | A 13 % Chromat. B 5.7 % Chromat. C 67 % Chromat. |

-
-
118537-84-1
2,3-dichlorobenzoic acid sodium salt

-
-
50-45-3
2,3-dichlorbenzoic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water pH=1 - 2; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 70 percent H2SO4 / 70 °C 2: sodium peroxide / H2O / 1 h / 20 °C View Scheme | |
| Stage #1: 2,3-dichlorobenzonitrile With sodium hydroxide; water In methanol at 5 - 10℃; for 10h; Heating / reflux; Stage #2: With hydrogenchloride |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: NaNO2; aq. HCl 1.2: copper(I) cyanide / H2O / 0.33 h / 20 °C 2.1: 70 percent H2SO4 / 70 °C 3.1: sodium peroxide / H2O / 1 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: MoCl5 / beim Chlorieren 2: alkaline permanganate View Scheme |
-
-
29027-17-6
2-chloro-3-methylaniline

-
-
50-45-3
2,3-dichlorbenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Diazotization.Behandlung der Diazoniumchloridloesung mit Cuprochlorid 2: diluted nitric acid / 140 °C / im Druckrohr View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: MoCl5 / beim Chlorieren 2: alkaline permanganate View Scheme | |
| Multi-step reaction with 2 steps 1: MoCl5 / beim Chlorieren 2: diluted nitric acid / 140 °C / im Druckrohr View Scheme |
-
-
21352-09-0
N-(2-chloro-6-methylphenyl)acetamide

-
-
50-45-3
2,3-dichlorbenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (i) aq. KMnO4, (ii) aq. H2SO4 2: (i) NaNO2, aq. HCl, (ii) aq. CuSO4, NH2OH*HCl, (iii) FeCl3, aq. HCl View Scheme |

| Conditions | Yield |
|---|---|
| With water In chlorobenzene at 28℃; Equilibrium constant; |
-
-
773837-37-9
sodium cyanide

-
-
2905-60-4
2,3-dichlorobenzoyl chloride

-
A
-
77668-42-9
2,3-dichlorobenzoyl cyanide

-
B
-
252186-80-4
2,3-dichlorobenzoic acid anhydride

-
C
-
50-45-3
2,3-dichlorbenzoic acid

| Conditions | Yield |
|---|---|
| With copper(l) chloride In para-xylene; acetonitrile Reagent/catalyst; Solvent; |


| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dichlorbenzoic acid With N,N-dimethyl-formamide In dichloromethane at 0℃; for 0.0833333h; Stage #2: With oxalyl dichloride In dichloromethane at 20℃; for 12h; | 100% |
| With thionyl chloride for 2h; Inert atmosphere; Reflux; | 88% |
| With thionyl chloride |
-
-
50-45-3
2,3-dichlorbenzoic acid


| Conditions | Yield |
|---|---|
| With 1-hydroxybenzotriazol-hydrate; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In acetonitrile at 20℃; | 100% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In water; acetonitrile at 20℃; | 100% |
-
-
50-45-3
2,3-dichlorbenzoic acid

| Conditions | Yield |
|---|---|
| With 2-chloro-1-methyl-pyridinium iodide; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 25℃; for 0.5h; Inert atmosphere; | 97% |
-
-
50-45-3
2,3-dichlorbenzoic acid

-
-
56529-85-2
2,2,2-trichloroethyl salicylate

-
-
88875-67-6
2,3-Dichloro-benzoic acid 2-(2,2,2-trichloro-ethoxycarbonyl)-phenyl ester

| Conditions | Yield |
|---|---|
| With methanesulfonyl chloride In pyridine; toluene for 10h; Ambient temperature; | 96% |

| Conditions | Yield |
|---|---|
| With sulfuric acid In methanol Reflux; | 92% |
| With sulfuric acid In methanol | 92% |

| Conditions | Yield |
|---|---|
| With sodium chlorite; potassium iodide at 100℃; for 24h; Schlenk technique; | 88% |

| Conditions | Yield |
|---|---|
| With copper; potassium carbonate In N,N-dimethyl-formamide at 25℃; for 0.25h; sonication; | 86% |
| With copper Ullmann condensation; Sonication; |
-
-
50-45-3
2,3-dichlorbenzoic acid

| Conditions | Yield |
|---|---|
| With caesium carbonate In dichloromethane at 20℃; for 16h; Inert atmosphere; Schlenk technique; | A n/a B 86% |


| Conditions | Yield |
|---|---|
| With pyridine; copper; potassium carbonate In water for 2h; Heating; | 85% |
| With copper(l) iodide; copper; potassium carbonate at 180℃; under 7355.08 Torr; |
-
-
50-45-3
2,3-dichlorbenzoic acid

-
-
95-86-3
2,4-diaminophenol

-
-
339197-79-4
2-(2,3-dichlorophenyl)benzo[d]oxazol-5-amine

| Conditions | Yield |
|---|---|
| With polyphosphoric acid at 110 - 120℃; for 16h; | 83% |
-
-
50-45-3
2,3-dichlorbenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dichlorbenzoic acid With potassium fluoride In tetrahydrofuran Large scale; Stage #2: With 2,2,6,6-tetramethylpiperidinyl-lithium at -78 - -70℃; for 1.5h; Large scale; Stage #3: With Triisopropyl borate In tetrahydrofuran at -78 - -70℃; for 1.16667h; Large scale; | 82.3% |

| Conditions | Yield |
|---|---|
| With ammonia; copper(l) chloride In methanol at 130℃; under 22801.5 Torr; for 20h; Pressure; Solvent; Autoclave; | 81% |
| With hydrogenchloride; sodium hydroxide; aqueous NH3; ammonia; copper(l) chloride In water | |
| With sodium hydroxide; ammonia; copper(l) chloride In water |

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dichlorbenzoic acid With thionyl chloride In benzene Heating / reflux; Stage #2: ethene With aluminum (III) chloride In 1,1-dichloroethane at 10 - 20℃; Stage #3: With aluminum (III) chloride; sodium chloride at 130 - 180℃; for 2h; | 80% |
-
-
50-45-3
2,3-dichlorbenzoic acid

| Conditions | Yield |
|---|---|
| With pyridine; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride at 70℃; for 2h; | 80% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2 at 60℃; for 16h; Inert atmosphere; | 80% |
-
-
50-45-3
2,3-dichlorbenzoic acid

-
-
114-33-0
N-Methylnicotinamide

-
-
7732-18-5
water

-
-
6046-93-1
copper(II) acetate monohydrate

| Conditions | Yield |
|---|---|
| for 72h; | 79% |

-
-
50-45-3
2,3-dichlorbenzoic acid

| Conditions | Yield |
|---|---|
| With triethylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In dichloromethane at 25℃; for 10h; Reagent/catalyst; | 76% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dichlorbenzoic acid; 2-Methoxybenzoic acid With manganese(IV) oxide; di-μ-chlorobis(norbornadiene)dirhodium(I) In water at 150℃; for 24h; Sealed tube; Stage #2: methyl iodide With potassium carbonate In water; acetone at 60℃; for 24h; regiospecific reaction; | 76% |

-
-
50-45-3
2,3-dichlorbenzoic acid

-
-
98175-84-9
1-phenyl-4,5,6,7-tetrahydro-1H-[1,2,3]triazolo[4,5-c]pyridine

| Conditions | Yield |
|---|---|
| With triethylamine; HATU In dichloromethane | 75% |
-
-
50-45-3
2,3-dichlorbenzoic acid

-
-
1266359-97-0
(4-benzothiazol-2-yl-piperazin-1-yl)acetic acid hydrazide

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dichlorbenzoic acid; (4-benzothiazol-2-yl-piperazin-1-yl)acetic acid hydrazide With 2,6-dimethylpyridine In dichloromethane at 25 - 30℃; for 0.5h; Stage #2: With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate In dichloromethane at 0 - 5℃; | 75% |
-
-
50-45-3
2,3-dichlorbenzoic acid

-
-
87673-89-0
1-phenyl-4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridine

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 0.5h; Inert atmosphere; | 75% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dichlorbenzoic acid With silica gel; trichlorophosphate at 20℃; for 0.5h; Stage #2: 4-methyl-2-(pyridine-3-yl)thiazole-5-carbohydrazide at 100℃; for 5h; | 74% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dichlorbenzoic acid With trichloroisocyanuric acid; triphenylphosphine In dichloromethane at 20℃; for 0.5h; Stage #2: 1,2,3-Benzotriazole In dichloromethane at 20℃; | 74% |
-
-
50-45-3
2,3-dichlorbenzoic acid

-
-
19335-11-6
1H-indazol-5-ylamine

| Conditions | Yield |
|---|---|
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; | 73% |
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; |
-
-
50-45-3
2,3-dichlorbenzoic acid

-
-
17287-03-5
trimethylsulphonium hydroxide

-
-
2905-54-6
2,3-Dichlorobenzoic acid methyl ester

| Conditions | Yield |
|---|---|
| In ethyl acetate at 20℃; for 2h; Esterification; | 72% |
-
-
50-45-3
2,3-dichlorbenzoic acid

-
-
1216936-53-6
3-(4-amino-5-mercapto-4H-1,2,4-triazol-3-yl)-1-ethyl-7-methyl-1,8-naphthyridin-4(1H)-one

-
-
1334936-30-9
3-{6-(2,3-dichlorophenyl)-1,2,4-triazolo[3,4-b][1,3,4]thiadiazol-3-yl}-1-ethyl-7-methyl-1,8-naphthyridin-4(1H)-one

| Conditions | Yield |
|---|---|
| Stage #1: 3-(4-amino-5-mercapto-4H-1,2,4-triazol-3-yl)-1-ethyl-7-methyl-1,8-naphthyridin-4(1H)-one With phosphoric acid at 50 - 60℃; Stage #2: 2,3-dichlorbenzoic acid at 180 - 200℃; for 3h; Stage #3: With ammonia In water | 71% |
-
-
54-96-6
3,4-diaminopyridine

-
-
50-45-3
2,3-dichlorbenzoic acid

-
-
1196131-11-9
2-(2,3-dichlorophenyl)-5H-imidazo[4,5-c]pyridine

| Conditions | Yield |
|---|---|
| With PPA at 190℃; for 3h; | 70% |
2,3-Dichlorobenzoic acid Standards and Recommendations
Content≥99%
2,3-Dichlorobenzoic acid Specification
The CAS register number of 2,3-Dichlorobenzoic acid is 50-45-3. It also can be called as Benzoic acid, 2,3-dichloro- and the IUPAC name about this chemical is 2,3-dichlorobenzoic acid. The molecular formula about this chemical is C7H4Cl2O2 and the molecular weight is 191.01. It belongs to the following product categories, such as Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts; Benzoic acid; Organic acids; Alpha sort; D; DAlphabetic; DIA - DIC; Pesticides & Metabolites; C7; Carbonyl Compounds; Carboxylic Acids and so on. This chemical can be used as intermediate of medicine and the intermediates of pesticide. If you want to store this chemical, plese keep it in a tightly closed container and store it in a cool, dry, well-ventilated area away from incompatible substances.
Physical properties about 2,3-Dichlorobenzoic acid are: (1)ACD/LogP: 2.92; (2)ACD/LogD (pH 5.5): 0.17; (3)ACD/LogD (pH 7.4): -0.22; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 1.63; (7)ACD/KOC (pH 7.4): 1; (8)#H bond acceptors: 2; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 1; (11)Polar Surface Area: 26.3Å2; (12)Index of Refraction: 1.598; (13)Molar Refractivity: 42.97 cm3; (14)Molar Volume: 125.8 cm3; (15)Polarizability: 17.03x10-24cm3; (16)Surface Tension: 53.9 dyne/cm; (17)Enthalpy of Vaporization: 57.8 kJ/mol; (18)Boiling Point: 306.9 °C at 760 mmHg; (19)Vapour Pressure: 0.000326 mmHg at 25°C.
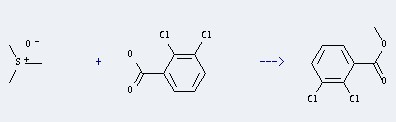
Uses of 2,3-Dichlorobenzoic acid: it can be used to produce 2,3-Dichlorobenzoic acid methyl ester with trimethylsulfonium; hydroξde at temperature of 20 °C. This reaction is a kind of Esterification. It will need solvent ethyl acηte with reaction time of 2 hour(s). The yield is about 72%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. When you are using it, wear suitable protective clothing, gloves and eye/face protection, you also need avoid contact with skin and eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: Clc1c(C(=O)O)cccc1Cl
(2)InChI: InChI=1/C7H4Cl2O2/c8-5-3-1-2-4(6(5)9)7(10)11/h1-3H,(H,10,11)
(3)InChIKey: QAOJBHRZQQDFHA-UHFFFAOYAD
(4)Std. InChI: InChI=1S/C7H4Cl2O2/c8-5-3-1-2-4(6(5)9)7(10)11/h1-3H,(H,10,11)
(5)Std. InChIKey: QAOJBHRZQQDFHA-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | subcutaneous | 900mg/kg (900mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: MUSCLE WEAKNESS LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Biochemical Pharmacology. Vol. 13, Pg. 1538, 1964. |
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 5045-40-9
- 50456-56-9
- 504-60-9
- 50461-74-0
- 50461-86-4
- 504-63-2
- 50463-25-7
- 50468-56-9
- 5047-01-8
- 50471-44-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View