-
Name
2,4,6-Trinitrotoluene
- EINECS 204-289-6
- CAS No. 118-96-7
- Article Data54
- CAS DataBase
- Density 1.608 g/cm3
- Solubility
- Melting Point 80.9 °C
- Formula C7H5N3O6
- Boiling Point 339.227 °C at 760 mmHg
- Molecular Weight 227.133
- Flash Point 167.109 °C
- Transport Information UN 0209
- Appearance yellow crystals
- Safety 35-45-61-36/37-26
- Risk Codes 2-23/24/25-33-51/53-36-20/21/22-11-1
-
Molecular Structure
-
Hazard Symbols
 E,
E,  T,
T,  N,
N,  Xn,
Xn,  F,
F,  B
B
- Synonyms Toluene,2,4,6-trinitro- (7CI,8CI);1-Methyl-2,4,6-trinitrobenzene;2-Methyl-1,3,5-trinitrobenzene;4-Methyl-1,3,5-trinitrobenzene;Gradetol;NSC 36949;TNT;Trinitrotoluene;Tritol(explosive);Trotyl;Trotyl oil;sym-Trinitrotoluene;sym-Trinitrotoluol;a-TNT;
- PSA 137.46000
- LogP 3.28920
Synthetic route

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid at 110℃; for 4h; Temperature; | 92.2% |
| With sulfuric acid; nitric acid at 20 - 100℃; Nitration; | 89% |
| With sulfuric acid; nitric acid 1.) 0 deg C, 10 min, 2.) 95 deg C, 6 h, then room temp., 12 h; | 69% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid at 110℃; for 4h; | 92.2% |
| With sulfuric acid; nitric acid | |
| durch Nitrierung; |

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid at 20 - 90℃; Nitration; | 84% |
| With nitric acid; Petroleum ether azeotropes Abdestillieren des gebildeten Wassers; | |
| With sulfuric acid; nitric acid |

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid 1.) 90-100 deg C, 2 h, 2.) 25 deg C, overnight; | 62% |
| Nitrierung; | |
| durch Nitrierung; |

| Conditions | Yield |
|---|---|
| With lead(IV) acetate; acetic acid |

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid at 60 - 70℃; |

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid at 93 - 103℃; | |
| With ammonium nitrate In sulfuric acid at 0 - 25℃; |
-
-
77601-83-3
2,4,6-trinitrophenylacetic acid

-
-
118-96-7
2,4,6-Trinitrotoluene

| Conditions | Yield |
|---|---|
| With water | |
| With ethanol |
-
-
99-65-0
meta-dinitrobenzene

-
-
546-67-8
lead(IV) tetraacetate

-
-
64-19-7
acetic acid

-
A
-
118-96-7
2,4,6-Trinitrotoluene

-
B
-
632-92-8
2,4-dimethyl-1,3,5-trinitrobenzene

| Conditions | Yield |
|---|---|
| Erwaermen des Reaktionsprodukts mit rauchender Salpetersaeure und konz. H2SO4 auf 75-120grad; |

-
-
99-35-4
1,3,5-trinitrobenzene

-
-
546-67-8
lead(IV) tetraacetate

-
-
64-19-7
acetic acid

-
A
-
118-96-7
2,4,6-Trinitrotoluene

-
B
-
632-92-8
2,4-dimethyl-1,3,5-trinitrobenzene


| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid Thermodynamic data; ΔH, E(a); |
-
-
121-14-2
2,4-dinitrotoluene

-
A
-
118-96-7
2,4,6-Trinitrotoluene

-
B
-
18242-38-1
1-bromo-2-methyl-3,5-dinitrobenzene

| Conditions | Yield |
|---|---|
| With sulfuric acid; bromine; nitric acid at 90℃; for 0.166667h; Title compound not separated from byproducts; | A 0.4 % Chromat. B 94.7 % Chromat. |
-
-
121-14-2
2,4-dinitrotoluene

-
A
-
118-96-7
2,4,6-Trinitrotoluene

-
B
-
18242-38-1
1-bromo-2-methyl-3,5-dinitrobenzene

-
C
-
96-90-2
1-chloro-2-methyl-3,5-dinitrobenzene

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid; chlorine at 90℃; for 0.8h; Rate constant; Mechanism; Product distribution; further reaction times; oleum instead of sulphuric acid; | A 19.1 % Chromat. B 8.8 % Chromat. C 52.3 % Chromat. |


| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 25℃; Equilibrium constant; Rate constant; |

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 25℃; Equilibrium constant; Rate constant; |

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 25℃; Equilibrium constant; Rate constant; |

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 25℃; Equilibrium constant; Rate constant; |

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 25℃; Equilibrium constant; Rate constant; |

| Conditions | Yield |
|---|---|
| With Isopropylamine hydroperchlorate In dimethyl sulfoxide at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| With n-butylammonium perchlorate In dimethyl sulfoxide at 25℃; Equilibrium constant; |

-
-
118-96-7
2,4,6-Trinitrotoluene

| Conditions | Yield |
|---|---|
| With sodium sulfite In water at 25℃; Equilibrium constant; variation with solvent composition; |

| Conditions | Yield |
|---|---|
| With piperidine hydrochloride In dimethyl sulfoxide at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| With benzylamine hydrogenperchlorate In dimethyl sulfoxide at 25℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| In acetonitrile at 25℃; Rate constant; different solvents; |

| Conditions | Yield |
|---|---|
| In methanol; dimethyl sulfoxide at 25℃; Rate constant; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| unter azeotropem Abdestillieren des entstehenden Wassers; | |
| in der Waerme bei groesserer Konzentration der Salpetersaeure; | |
| unter azeotropem Abdestillieren des entstehenden Wassers; |

| Conditions | Yield |
|---|---|
| in der Waerme; |

-
-
99-87-6
4-methylisopropylbenzene

-
-
7664-93-9
sulfuric acid

-
-
7697-37-2
nitric acid

-
A
-
118-96-7
2,4,6-Trinitrotoluene

-
B
-
16533-71-4
3,5-dinitro-p-toluic acid

| Conditions | Yield |
|---|---|
| at 60 - 70℃; |


| Conditions | Yield |
|---|---|
| With piperidine In benzene for 12h; Knoevenagel Condensation; Dean-Stark; Reflux; | 95.1% |
-
-
118-96-7
2,4,6-Trinitrotoluene

-
-
35113-75-8
1-nitromethyl-2,4,6-trinitrobenzene

| Conditions | Yield |
|---|---|
| With potassium hydroxide; fluorotrinitromethane In tetrahydrofuran; methanol; water for 0.0333333h; Nitration; | 95% |
-
-
118-96-7
2,4,6-Trinitrotoluene

| Conditions | Yield |
|---|---|
| Stage #1: 2,4,6-Trinitrotoluene With hydrogen; pyrographite In methanol at 120℃; under 45004.5 Torr; for 5h; Large scale; Stage #2: With hydrogenchloride In water for 1h; Reagent/catalyst; Solvent; Pressure; Large scale; | 95% |
-
-
118-96-7
2,4,6-Trinitrotoluene

-
-
606-34-8
2,4,6-trinitrobenzaldehyde

-
-
20062-22-0
2,2’,4,4’,6,6’-hexanitrostilbene

| Conditions | Yield |
|---|---|
| With piperidine In toluene Reflux; | 94.2% |
-
-
118-96-7
2,4,6-Trinitrotoluene

| Conditions | Yield |
|---|---|
| Stage #1: 2,4,6-Trinitrotoluene With hydrogen In toluene at 120℃; under 30003 Torr; for 10h; Autoclave; Stage #2: With hydrogenchloride In water for 0.5h; Solvent; Pressure; Temperature; | 93.1% |

| Conditions | Yield |
|---|---|
| With piperidine In benzene for 14h; Knoevenagel Condensation; Dean-Stark; Reflux; | 90.3% |

| Conditions | Yield |
|---|---|
| With piperidine In benzene for 6h; Knoevenagel Condensation; Dean-Stark; Reflux; | 90.2% |
-
-
1125-80-0
3-methylisoquinoline

-
-
118-96-7
2,4,6-Trinitrotoluene

-
-
98-88-4
benzoyl chloride

-
-
94170-03-3
[3-Methyl-1-(2,4,6-trinitro-benzyl)-1H-isoquinolin-2-yl]-phenyl-methanone

| Conditions | Yield |
|---|---|
| In chloroform at 60 - 70℃; for 1h; | 90% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride; palladium on activated charcoal; hydrogen In methanol at 20℃; under 18751.9 Torr; for 2h; Solvent; Pressure; Time; | 90% |
| With iron(III) chloride; pyrographite; hydrazine hydrate In methanol for 7h; Heating; | 67.8% |
-
-
118-96-7
2,4,6-Trinitrotoluene

-
-
544676-41-7
3-formyl-4,6-dinitro-1-phenyl-1H-indazole

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol at 20℃; for 24h; | 90% |

| Conditions | Yield |
|---|---|
| 90% | |
| In toluene at 20℃; for 24h; | 70% |

| Conditions | Yield |
|---|---|
| With piperidine In benzene for 11h; Knoevenagel Condensation; Dean-Stark; Reflux; | 89.5% |

| Conditions | Yield |
|---|---|
| With sodium chlorate; nitric acid | 88% |
| With sodium dichromate; sulfuric acid at 45 - 55℃; for 2h; Oxidation; | 14% |
| With sodium chlorate; nitric acid Reinigung ueber das Natrium-Salz; |
-
-
118-96-7
2,4,6-Trinitrotoluene

-
-
100-52-7
benzaldehyde

-
-
61599-68-6
1,3,5-trinitro-2-[(E)-2-phenylvinyl]benzene

| Conditions | Yield |
|---|---|
| With piperidine; silica gel In neat (no solvent) at 120℃; for 0.333333h; Microwave irradiation; | 88% |
| With piperidine In benzene for 6h; Condensation; Heating; | 81% |
| With HTc-4-Cal In toluene for 15h; Reagent/catalyst; Time; Reflux; Dean-Stark; | 70% |
| With piperidine | |
| With piperidine; ethanol at 40℃; |
-
-
118-96-7
2,4,6-Trinitrotoluene

-
-
2365-48-2
Methyl thioglycolate

-
A
-
321596-17-2
2,4-dinitro-6-[(methoxycarbonyl)methylthio]toluene

| Conditions | Yield |
|---|---|
| With potassium carbonate In 1-methyl-pyrrolidin-2-one at 20℃; for 24h; | A 88% B n/a |

-
-
118-96-7
2,4,6-Trinitrotoluene

-
-
104-88-1
4-chlorobenzaldehyde

-
-
65200-05-7
2-[(E)-2-(4-Chloro-phenyl)-vinyl]-1,3,5-trinitro-benzene

| Conditions | Yield |
|---|---|
| With piperidine In benzene for 5.5h; Knoevenagel Condensation; Dean-Stark; Reflux; | 87.6% |

| Conditions | Yield |
|---|---|
| With piperidine In benzene for 6h; Knoevenagel Condensation; Dean-Stark; Reflux; | 87% |
-
-
19788-49-9
Ethyl 2-mercaptopropionate

-
-
118-96-7
2,4,6-Trinitrotoluene

| Conditions | Yield |
|---|---|
| With potassium carbonate In 1-methyl-pyrrolidin-2-one at 20℃; for 24h; | A 86% B n/a |

-
-
118-96-7
2,4,6-Trinitrotoluene

-
-
52886-05-2
2,4,6-trinitro(α,αα-D3)toluene

| Conditions | Yield |
|---|---|
| With tributyl-amine; water-d2 In N,N-dimethyl-formamide for 5h; | 85% |
| With [(2)H6]acetone; deuteromethanol; water-d2; triethylamine for 1.5h; Ambient temperature; | 63% |
-
-
118-96-7
2,4,6-Trinitrotoluene

-
-
540-63-6
ethane-1,2-dithiol

-
-
1608182-18-8
1,2-bis((2-methyl-3,5-dinitrophenyl)thio)ethane

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 2h; | 85% |
-
-
118-96-7
2,4,6-Trinitrotoluene

-
-
623-51-8
ethyl 2-sulfanylacetate

-
-
367925-03-9
(2-methyl-3,5-dinitro-phenylsulfanyl)-acetic acid ethyl ester

| Conditions | Yield |
|---|---|
| With alkaline resin In acetone at 50℃; for 20h; Large scale; | 85% |
-
-
118-96-7
2,4,6-Trinitrotoluene

-
-
123-11-5
4-methoxy-benzaldehyde

-
-
61599-69-7
2-[(E)-2-(4-methoxyphenyl)vinyl]-1,3,5-trinitrobenzene

| Conditions | Yield |
|---|---|
| With HTc-4-Cal In toluene for 24h; Knoevenagel Condensation; Reflux; Dean-Stark; | 83% |
| With piperidine In benzene for 6h; Condensation; Heating; | 60% |
| With piperidine |

| Conditions | Yield |
|---|---|
| With piperidine; silica gel In neat (no solvent) at 120℃; for 0.333333h; Microwave irradiation; | 83% |
| With HTc-4-Cal In toluene for 24h; Knoevenagel Condensation; Reflux; Dean-Stark; | 71% |
-
-
107-03-9
1-thiopropane

-
-
118-96-7
2,4,6-Trinitrotoluene

-
-
1608182-14-4
(2-methyl-3,5-dinitrophenyl)(propyl)sulfane

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 2h; | 83% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 83% |

| Conditions | Yield |
|---|---|
| With piperidine; silica gel In neat (no solvent) at 120℃; for 0.333333h; Microwave irradiation; | 82% |
| With piperazine at 80 - 90℃; |
-
-
118-96-7
2,4,6-Trinitrotoluene

-
-
104-88-1
4-chlorobenzaldehyde

-
-
61599-70-0
trans-4′-chloro-2,4,6-trinitrostilbene

| Conditions | Yield |
|---|---|
| With piperidine; silica gel In neat (no solvent) at 120℃; for 0.333333h; Microwave irradiation; | 82% |
| With HTc-4-Cal In toluene for 24h; Knoevenagel Condensation; Reflux; Dean-Stark; | 70% |
| With piperidine at 120℃; |
2,4,6-TRINITROTOLUENE Chemical Properties
Molar mass: 227.13 g/mol
Appearance: Pale yellow. Loose "needles" before melt-casting. A solid block after being poured into a casing.
Density: 1.654 g/cm3
Melting point: 80.35 °C
Boiling point: 295 °C (decomposition)
Solubility in water: 0.13 g/L (20 °C)
Solubility in ether, acetone, benzene, pyridine: soluble
Other names:1,3,5-trinitrotoluene, sym-trinitrotoluene, TNT.
The Structure of 2,4,6-trinitrotoluene(118-96-7):
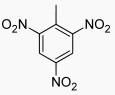
2,4,6-TRINITROTOLUENE History
2,4,6-trinitrotoluene(118-96-7) was first prepared in 1863 by German chemist Joseph Wilbrand and originally used as a yellow dye. Its potential as an explosive was not appreciated for several years mainly because it was so difficult to detonate and because it was less powerful than alternatives. TNT can be safely poured when liquid into shell cases, and is so insensitive that in 1910, it was exempted from the UK's Explosives Act 1875 and was not considered an explosive for the purposes of manufacture and storage.
The German armed forces adopted it as a filling for artillery shells in 1902. TNT-filled armour-piercing shells would explode after they had penetrated the armour of British capital ships, whereas the British lyddite-filled shells tended to explode upon striking armour, thus expending much of their energy outside the ship. The British started replacing lyddite with TNT in 1907. TNT is still widely used by the United States military and construction companies around the world. The majority of TNT currently used by the US military is manufactured by Radford Army Ammunition Plant near Radford, Virginia.
2,4,6-TRINITROTOLUENE Uses
2,4,6-trinitrotoluene(118-96-7) is one of the most commonly used explosives for military and industrial applications.
2,4,6-TRINITROTOLUENE Production
Three-stage nitration to mono-, di-, and trinitrotoluene was formerly used, but continuous-flow stirred-tank reactors and tubular units using the countercurrent flow of strong acids and toluene permit better yields and reaction control.
2,4,6-TRINITROTOLUENE Toxicity Data With Reference
| 1. | skn-rbt 500 mg/24H MLD | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) AD-B011-150 . | ||
| 2. | mmo-sat 10 µg/plate | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) AD-A080-146 . | ||
| 3. | orl-hmn LDLo:28 g/kg:CNS,PUL,GIT | 34ZIAG Toxicology of Drugs and Chemicals ,Deichmann, W.B.,New York, NY.: Academic Press, Inc.,1969,610. | ||
| 4. | orl-rat LD50:795 mg/kg | JTEHD6 Journal of Toxicology and Environmental Health. 9 (1982),565. | ||
| 5. | orl-mus LD50:660 mg/kg | JTEHD6 Journal of Toxicology and Environmental Health. 9 (1982),565. | ||
| 6. | orl-cat LDLo:1850 mg/kg | MRCSAB Medical Research Council, Special Report Series. 58 (1921),32. | ||
| 7. | scu-cat LDLo:200 mg/kg | MRCSAB Medical Research Council, Special Report Series. 58 (1921),32. | ||
| 8. | orl-rbt LDLo:500 mg/kg | MRCSAB Medical Research Council, Special Report Series. 58 (1921),32. | ||
| 9. | scu-rbt LDLo:500 mg/kg | MRCSAB Medical Research Council, Special Report Series. 58 (1921),32. |
2,4,6-TRINITROTOLUENE Consensus Reports
2,4,6-TRINITROTOLUENE Safety Profile
Flammable or explosive when exposed to heat or flame. Moderate explosion hazard; will detonate under strong shock. It detonates at around 240°C but can be distilled safely under reduced pressure. It is a comparatively insensitive explosive. In small quantities it will burn quietly if not confined. However, sudden heating of any quantity will cause it to detonate; the accumulation of heat when large quantities are burning will cause detonation. In other respects it is one of the most stable of all high explosives, and there are but a few restrictions for its handling. It is for this reason, from the military standpoint, that TNT is quantitatively the most used. It requires a fall of 130 cm for a 2 kg weight to detonate it. It is one of the most powerful high explosives. It can be detonated by the usual detonators and blasting caps (at least a No. 6). For full efficiency, the use of a high-velocity initiator, such as tetryl, is required. TNT is one of those explosives containing an oxygen deficiency. In other words, the addition of products that are oxygen rich can enhance its explosive power. Also mono- and dinitrotoluene may be added for reduction of the temperature of the explosion and to make the explosion flashless. Various materials are added to TNT to make what are known as permissible explosives. TNT may be regarded as the equivalent of 40% dynamite and can be used underwater. It is also used in the manufacture of a detonator fuse known as cordeau detonant. For the military, TNT finds use in all types of bursting charges, including armor-piercing types, although it is somewhat too sensitive to be ideal for this purpose and has since been replaced to a great extent by ammonium picrate. It is a relatively expensive explosive and does not compete seriously with dynamite for general commercial use.
Highly dangerous; explodes with shock or heating to 297°C. Various materials can reduce the explosive temperature: red lead (to 192°C), sodium carbonate (to 218°C), potassium hydroxide (to 192°C). Mixtures with sodium dichromate + sulfuric acid may ignite spontaneously. Reacts with nitric acid + metals (e.g., lead or iron) to form explosive products more sensitive to shock, friction, or contact with nitric or sulfuric acids. Reacts with potassium hydroxide dissolved in methanol to form explosive aci-nitro salts. Bases (e.g., sodium hydroxide, potassium iodide, tetramethyl ammonium octahydrotriborate) induce deflagration in molten TNT. Can react vigorously with reducing materials. When heated to decomposition it emits highly toxic fumes of NOx. See also NITRO COMPOUNDS of AROMATIC HYDROCARBONS and EXPLOSIVES, HIGH.
2,4,6-TRINITROTOLUENE Standards and Recommendations
ACGIH TLV: TWA 0.1 ppm
DFG MAK: 0.011 ppm (0.1 mg/m3); Confirmed Animal Carcinogen with Unknown Relevance to Humans
DOT Classification: EXPLOSIVE 1.1D; Label: EXPLOSIVE 1.1D (UN 0209); DOT Class: 4.1; Label: Flammable Solid (UN 1356)
2,4,6-TRINITROTOLUENE Analytical Methods
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 1189671-27-9
- 118969-27-0
- 118969-29-2
- 118971-00-9
- 118971-03-2
- 1189-71-5
- 118971-61-2
- 118-97-8
- 1189801-51-1
- 1189805-39-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View