-
Name
2-Aminopyrimidine
- EINECS 203-648-4
- CAS No. 109-12-6
- Article Data71
- CAS DataBase
- Density 1.216 g/cm3
- Solubility soluble in water
- Melting Point 122-126 °C(lit.)
- Formula C4H5N3
- Boiling Point 275.1 °C at 760 mmHg
- Molecular Weight 95.1038
- Flash Point 144.5 °C
- Transport Information UN 2811
- Appearance white to light yellow powder
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms pyrimidin-2-amine;2-Pyrimidiylamine;Pyrimidine, 2-amino-;2-Pyrimidinamine;2-Amino-pyrimidine;pyrimidin-2-ylamine;
- PSA 51.80000
- LogP 0.64000
Synthetic route

| Conditions | Yield |
|---|---|
| 97% |

| Conditions | Yield |
|---|---|
| With copper(I) oxide; potassium carbonate; ammonium hydroxide; N,N-dimethylethylenediamine In ethylene glycol at 60℃; for 16h; Inert atmosphere; | 94% |
-
-
79917-54-7
nitro-pyrimidine

-
-
109-12-6
2-aminopyrimidine

| Conditions | Yield |
|---|---|
| With triethylamine In water at 80℃; for 8h; Inert atmosphere; Green chemistry; chemoselective reaction; | 90% |
| With sodium tetrahydroborate In methanol; water at 40℃; for 0.5h; Green chemistry; |
-
-
1722-12-9
2-chloropyrimidine

-
-
51689-86-2
O-potassio-2,4-dimethyl-3-pentanone

-
A
-
109-12-6
2-aminopyrimidine

-
B
-
75782-24-0
2,4-Dimethyl-2-pyrimidin-2-yl-pentan-3-one

-
C
-
75782-25-1
2-(2-chloropyrimidin-4-yl)-2,4-dimethyl-3-pentanone

| Conditions | Yield |
|---|---|
| With ammonia In diethyl ether for 0.25h; Product distribution; Mechanism; Irradiation; effect of inhibitor (di-tert-butyl-nitroxide) and exclusion of light; | A 4% B 88% C n/a |


| Conditions | Yield |
|---|---|
| With potassium amide In diethyl ether; ammonia for 0.25h; exclusion of light; | 88% |
| With ammonia at 20℃; Kinetics; | |
| With ammonia at 80℃; under 67506.8 Torr; Kinetics; Temperature; |

| Conditions | Yield |
|---|---|
| for 3h; Heating; | 74% |
-
-
275-03-6
tetrazolo[1,5-a]pyrimidine

-
-
71-43-2
benzene

-
A
-
109-12-6
2-aminopyrimidine

-
B
-
57356-49-7
phenyl-pyrimidin-2-yl-amine

-
C
-
92-52-4
biphenyl

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 25℃; for 8h; Irradiation; | A 11% B 66% C n/a |
| With trifluoroacetic acid at 25℃; for 8h; Product distribution; with/without heavy-atom solvent (CH2CCl2, CH2Br2); other solvent; other reag; other temp.; | A 11% B 66% C n/a |

-
-
1722-12-9
2-chloropyrimidine

-
-
128190-66-9, 25088-58-8
potassium enolate of acetone

-
A
-
109-12-6
2-aminopyrimidine

-
B
-
75782-22-8
1-(2-pyrimidinyl)-2-propanone

| Conditions | Yield |
|---|---|
| With ammonia In diethyl ether for 0.25h; Mechanism; Product distribution; Irradiation; effect of inhibitor (di-tert-butyl-nitroxide) and exclusion of light; | A 4% B 61% |

-
-
191487-39-5
pyrimidin-2-yl-carbamic acid benzyl ester

-
A
-
109-12-6
2-aminopyrimidine

-
B
-
108-53-2
isocytosine

| Conditions | Yield |
|---|---|
| With Rhodococcus erythropolis DSM 6138 In ethanol for 16h; | A n/a B n/a C 58% |

-
-
110526-12-0
2-(N-sulphinylamino)pyrimidine

-
-
1165952-91-9
cyclohexa-1,3-diene

-
A
-
109-12-6
2-aminopyrimidine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -75℃; for 22h; hetero-Diels-Alder reaction; | A n/a B 14% C 51% |

-
-
106-42-3
para-xylene

-
-
275-03-6
tetrazolo[1,5-a]pyrimidine

-
A
-
109-12-6
2-aminopyrimidine

-
B
-
721-45-9
1,4-dimethyl-2-(4-methylbenzyl)benzene

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 25℃; for 8h; Irradiation; | A 27% B 9.8% C 47% |

-
-
100-66-3
methoxybenzene

-
-
275-03-6
tetrazolo[1,5-a]pyrimidine

-
A
-
109-12-6
2-aminopyrimidine

-
B
-
137379-63-6
(4-methoxy-phenyl)-pyrimidin-2-yl-amine

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 25℃; for 6h; Product distribution; other reag; other reaction time; | A 5% B 33% C 40% |
| With trifluoroacetic acid at 25℃; for 8h; Irradiation; | A 20% B 24% C 28% |

-
-
275-03-6
tetrazolo[1,5-a]pyrimidine

-
-
108-88-3
toluene

-
A
-
109-12-6
2-aminopyrimidine

-
B
-
101-81-5
Diphenylmethane

-
C
-
198711-26-1
N-(4-tolyl)pyrimidin-2-amine

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 25℃; for 8h; Irradiation; Title compound not separated from byproducts; | A 18 % Chromat. B 11 % Chromat. C 19 % Chromat. D 39% |

-
-
275-03-6
tetrazolo[1,5-a]pyrimidine

-
-
108-88-3
toluene

-
A
-
109-12-6
2-aminopyrimidine

-
B
-
101-81-5
Diphenylmethane

-
C
-
198711-26-1
N-(4-tolyl)pyrimidin-2-amine

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 25℃; for 6h; Product distribution; other reag; other reaction time; | A 13% B 5% C 23% D 38% E 2.2% |

-
-
100-41-4
ethylbenzene

-
-
275-03-6
tetrazolo[1,5-a]pyrimidine

-
A
-
109-12-6
2-aminopyrimidine

-
B
-
6196-94-7, 71886-67-4
1-ethyl-4-(1-phenylethyl)benzene

-
C
-
18908-70-8
1-(o-ethylphenyl)-1-phenylethane

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 25℃; for 6h; Product distribution; other reag; other reaction time; | A 1% B n/a C n/a D 32% E 0.8% F 36% |

-
-
35034-15-2
2‐aminopyrimidine‐1‐oxide

-
A
-
109-12-6
2-aminopyrimidine

-
B
-
5428-89-7
5-chloropyrimidin-2-amine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite | A 34% B 8.5% C 6% |

-
-
108-90-7
chlorobenzene

-
-
275-03-6
tetrazolo[1,5-a]pyrimidine

-
A
-
109-12-6
2-aminopyrimidine

-
C
-
214627-66-4
(3-chloro-phenyl)-pyrimidin-2-yl-amine

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 25℃; for 8h; Irradiation; | A 34% B 6.2% C 0.4% D 21% |

-
-
106-42-3
para-xylene

-
-
275-03-6
tetrazolo[1,5-a]pyrimidine

-
A
-
109-12-6
2-aminopyrimidine

-
B
-
92-52-4
biphenyl

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 25℃; for 6h; Product distribution; with/without heavy-atom solvent (CH2CCl2, CH2Br2); other solvent; other reag; other reaction time; | A 19% B n/a C 34% |

-
-
1722-12-9
2-chloropyrimidine

-
-
351531-29-8
O-potassio-3,3-dimethyl-2-butanone

-
A
-
109-12-6
2-aminopyrimidine

-
B
-
75782-23-9
1-(2-pyrimidinyl)-3,3-dimethyl-2-butanone

| Conditions | Yield |
|---|---|
| With ammonia In diethyl ether for 0.25h; Mechanism; Product distribution; Irradiation; | A 4% B 32% |


| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 25℃; for 8h; Irradiation; Further byproducts given. Title compound not separated from byproducts; | A 27 % Chromat. B 14 % Chromat. C 4.8 % Chromat. D 21% |

-
-
65267-35-8
N-benzylidene-N'-pyrimidin-2-ylhydrazine

-
A
-
109-12-6
2-aminopyrimidine

-
B
-
22514-82-5
2,3-diphenyl quinoline

-
C
-
484-47-9
lophine

-
D
-
100-47-0
benzonitrile

-
E
-
55643-77-1
2-phenyl<1,2,4>triazolo<1,5-a>pyrimidine

-
F
-
65267-36-9
3-phenyl-[1,2,4]triazolo[4,3-a]pyrimidine

| Conditions | Yield |
|---|---|
| at 350℃; under 0.02 Torr; for 1h; Mechanism; Pyrolysis; | A 18% B 10% C 16% D 15% E 17% F 15% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride; ethanol |

| Conditions | Yield |
|---|---|
| With water; zinc | |
| With potassium hydroxide; palladium on activated charcoal Hydrogenation; | |
| With sodium hydroxide; palladium on activated charcoal; diethyl ether Hydrogenation; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With methanol; Lindlar's catalyst; barium(II) oxide Hydrogenation; | |
| With Lindlar's catalyst; ethanol; barium(II) oxide Hydrogenation; | |
| elektrochemischen Reduktion an einer Cadmium-Kathode; |

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; sulfuric acid | |
| With chromium(VI) oxide; sulfuric acid |
-
-
98021-69-3
acetic acid-(1-bromo-3,3-dichloro-propyl ester)

-
-
113-00-8
guanidine nitrate

-
-
109-12-6
2-aminopyrimidine

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; ammonium acetate In acetonitrile at 20℃; for 0.0833333h; | 100% |
| With N-Bromosuccinimide; ammonium acetate In acetonitrile at 20℃; for 0.5h; | 98% |
| With N-Bromosuccinimide In acetonitrile at 20℃; Cooling with ice; Darkness; | 97% |

| Conditions | Yield |
|---|---|
| In ethanol Reflux; | 100% |
| With sodium hydrogencarbonate for 16h; Ambient temperature; | 75% |
| With sodium hydrogencarbonate In ethanol; water for 48h; Heating; | |
| With sodium hydrogencarbonate In ethanol; water for 48h; Heating; |
-
-
109-12-6
2-aminopyrimidine

-
-
31949-21-0
bromomethyl 2-methoxyphenyl ketone

-
-
143696-71-3
2-(2-methoxyphenyl)imidazo[1,2-a]pyrimidine

| Conditions | Yield |
|---|---|
| In ethanol at 75℃; for 24h; | 100% |
| In ethanol Reflux; | 92% |
| In 1,2-dimethoxyethane for 48h; Heating; | |
| With sodium hydrogencarbonate In 2-methyltetrahydrofuran at 85℃; for 10h; Sealed tube; |
-
-
109-12-6
2-aminopyrimidine

-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
-
6578-34-3
N,N-dimethyl-N'-pyrimidin-2-ylformamidine

| Conditions | Yield |
|---|---|
| In toluene Heating; | 100% |
| for 6h; Reflux; | 80% |
| In toluene for 2h; Heating; |
-
-
109-12-6
2-aminopyrimidine

-
-
161868-74-2
1,1-dichloro-5-aza-2,8-dioxa-1-phosphaV-dibenzo<9,9',11,11'-tetra-tert-butyl>-bicyclo<3.3.0>octadiene

| Conditions | Yield |
|---|---|
| In benzene for 2h; Ambient temperature; | 100% |
-
-
109-12-6
2-aminopyrimidine

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 2h; Cyclization; Heating; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminopyrimidine; 1,1,1-Trifluoro-2,4-pentanedione Stage #2: With sulfuric acid | 100% |
-
-
109-12-6
2-aminopyrimidine

-
-
70-23-5
ethyl Bromopyruvate

-
-
149520-94-5
2-amino-1H-imidazole-4-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminopyrimidine; ethyl Bromopyruvate In ethanol at 75℃; for 16h; Inert atmosphere; Stage #2: With hydrazine hydrate In ethanol at 75℃; for 16h; Inert atmosphere; | 100% |
| Stage #1: 2-aminopyrimidine; ethyl Bromopyruvate In ethanol at 75℃; Stage #2: With hydrazine hydrate In ethanol at 75℃; | 54% |
| Stage #1: 2-aminopyrimidine; ethyl Bromopyruvate In ethanol at 75℃; for 18h; Stage #2: With hydrazine hydrate In ethanol at 75℃; for 16.5h; | 0.34 g |
-
-
109-12-6
2-aminopyrimidine

-
-
41078-65-3
1,4,5,6-tetrahydro-pyrimidin-2-ylamine

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen; acetic acid at 20℃; under 760.051 Torr; for 17h; Time; | 100% |

| Conditions | Yield |
|---|---|
| With iron(II,III) oxide; potassium tert-butylate In 1,4-dioxane at 90℃; for 168h; Inert atmosphere; | 99% |
| With potassium tert-butylate; copper diacetate In 1,4-dioxane at 130℃; for 48h; | 99% |
| With potassium tert-butylate; copper diacetate In 1,4-dioxane at 130℃; for 48h; Inert atmosphere; | 99% |
-
-
109-12-6
2-aminopyrimidine

-
-
612-57-7
6-chloroquinoline

-
-
1160861-67-5
N-(pyrimidin-2-yl)quinolin-6-amine

| Conditions | Yield |
|---|---|
| With chloro[2-(dicyclohexylphosphino)-3 ,6-dimethoxy-2’,4’, 6’-triisopropyl- 1,1’-biphenyl] [2-(2-aminoethyl)phenyl]palladium(II); caesium carbonate; ruphos In tert-butyl alcohol at 110℃; for 24h; Inert atmosphere; | 99% |
| With water; potassium carbonate; palladium diacetate; dicyclohexyl-(2′,4′,6′-triisopropyl-3,6-dimethoxy-[1,1′-biphenyl]-2-yl)phosphine In tert-butyl alcohol at 110℃; for 1h; Inert atmosphere; | 91% |
| With dicyclohexyl-(2′,4′,6′-triisopropyl-3,6-dimethoxy-[1,1′-biphenyl]-2-yl)phosphine; C47H63NO2PPd(2+)*CH3O3S(1-); sodium t-butanolate In 1,4-dioxane at 100℃; for 24h; | 90% |
-
-
109-12-6
2-aminopyrimidine

-
-
50-00-0
formaldehyd

-
-
131543-46-9
Glyoxal

-
-
134914-65-1
2-(1H-imidazol-1-yl)pyrimidine

| Conditions | Yield |
|---|---|
| With phosphoric acid; ammonium chloride In methanol Reflux; | 99% |


| Conditions | Yield |
|---|---|
| With pyridine In chloroform at 20℃; for 48h; chemoselective reaction; | 99% |
-
-
109-12-6
2-aminopyrimidine

| Conditions | Yield |
|---|---|
| With pyridine In chloroform at 20℃; for 48h; chemoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With C51H57ClNiO2P2; sodium t-butanolate at 25℃; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminopyrimidine; p-acetylaminobenzenesulfonyl chloride With calcium carbonate In toluene at 20 - 65℃; Stage #2: With water; sodium hydroxide In toluene at 100 - 115℃; Stage #3: With acetic acid pH=5 - 5.5; Reagent/catalyst; | 98.5% |
| Stage #1: 2-aminopyrimidine; p-acetylaminobenzenesulfonyl chloride With pyridine In tetrahydrofuran at 20℃; for 6h; Inert atmosphere; Stage #2: With sodium hydroxide for 2h; Inert atmosphere; Reflux; | 65% |
| Stage #1: 2-aminopyrimidine; p-acetylaminobenzenesulfonyl chloride With pyridine In tetrahydrofuran at 20℃; for 6h; Stage #2: With sodium hydroxide for 2h; Reflux; |
-
-
109-12-6
2-aminopyrimidine

-
-
10025-99-7
potassium tetrachloroplatinate(II)

-
-
60185-34-4, 64440-33-1, 82371-67-3
cis-[bis(2-aminopyrimidine)-dichloro-platinum]

| Conditions | Yield |
|---|---|
| In water for 48h; Darkness; | 98.36% |
| In water byproducts: KCl; addn. of pyrimidine soln. to Pt complex soln. (Pt:pyrimidine = 1:4), room temp.; holding, 10-12h; pptn.; filtration; repeated washing (water); drying (80°C); |

| Conditions | Yield |
|---|---|
| In toluene for 6h; Reflux; | 98% |
| at 60 - 70℃; Condensation; | |
| In toluene for 2h; | 88 mg |

| Conditions | Yield |
|---|---|
| Stage #1: ibuprofen With 1,1'-carbonyldiimidazole In dichloromethane at 20℃; for 0.5h; Stage #2: 2-aminopyrimidine In dichloromethane for 72h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; sodium t-butanolate In toluene at 95℃; for 18h; Buchwald-Hartwig Coupling; Schlenk technique; Inert atmosphere; | 98% |
| With copper(l) iodide; potassium carbonate; N,N`-dimethylethylenediamine In 1,4-dioxane at 100℃; for 22h; | 78% |
| With potassium tert-butylate In dimethyl sulfoxide at 120℃; for 12h; | 72% |
| With copper(l) iodide; potassium tert-butylate In 1,4-dioxane at 130℃; for 24h; Ullmann Condensation; Sealed tube; Inert atmosphere; | 42% |
-
-
109-12-6
2-aminopyrimidine

-
-
6642-31-5
6-Amino-1,3-dimethylbarbituric acid

-
-
1242060-34-9
6-amino-1,3-dimethyl-5-[(2-pyrimidyl)diazenyl]uracil

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminopyrimidine With sulfuric acid; acetic acid; sodium nitrite at 0 - 5℃; for 2h; Stage #2: 6-Amino-1,3-dimethylbarbituric acid With sodium hydroxide In water at 0 - 5℃; for 0.25h; pH=7; | 98% |
-
-
109-12-6
2-aminopyrimidine

-
-
873-76-7
para-Chlorobenzyl alcohol

-
-
23676-57-5
N-(4-chlorobenzyl)-(2-pyrimidyl)amine

| Conditions | Yield |
|---|---|
| With [RuCl(CO)(PPh3)(PNS-Me)]; potassium hydroxide In toluene at 100℃; for 12h; | 98% |
| With [N,S-(2-(2-(diphenylphosphino)benzylidene)-N-ethylthiosemicarbazone)RuH(CO)(PPh3)2]; potassium hydroxide In toluene at 100℃; for 12h; Reagent/catalyst; | 97% |
| With C41H36AsClN3OPRuS; potassium hydroxide In toluene at 100℃; for 12h; | 97% |
-
-
109-12-6
2-aminopyrimidine

-
-
2052-07-5
2-Bromobiphenyl

-
-
1372776-06-1
N-([1,1'-biphenyl]-2-yl)pyrimidin-2-amine

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; sodium t-butanolate In toluene at 95℃; for 18h; Buchwald-Hartwig Coupling; Schlenk technique; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With SingaCycle-A1; potassium hexamethylsilazane In toluene at 100℃; for 24h; Schlenk technique; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With pyridine at 0℃; for 2h; Inert atmosphere; | 97% |
-
-
109-12-6
2-aminopyrimidine

-
-
120-89-8
parabanic acid

-
-
123-11-5
4-methoxy-benzaldehyde

-
-
1089671-87-3
2-(4-methoxyphenyl)-N3-[(E)-1-(4-methoxyphenyl)methylidene]imidazo[1,2-a]pyrimidin-3-amine

| Conditions | Yield |
|---|---|
| at 200℃; for 0.0833333h; | 97% |

-
-
109-12-6
2-aminopyrimidine

-
-
120-89-8
parabanic acid

-
-
620-23-5
m-tolyl aldehyde

-
-
1089671-86-2
2-(3-methylphenyl)-N3-[(E)-1-(3-methylphenyl)methylidene]imidazo[1,2-a]pyrimidin-3-amine

| Conditions | Yield |
|---|---|
| at 200℃; for 0.0833333h; | 97% |

-
-
109-12-6
2-aminopyrimidine

-
-
104-87-0
4-methyl-benzaldehyde

-
-
135-19-3
β-naphthol

-
-
1094443-80-7
1-(p-methylphenyl(2-pyrimidinylamino)methyl)naphthalene-2-ol

| Conditions | Yield |
|---|---|
| In water at 20℃; for 0.416667h; | 97% |
| at 125℃; for 0.05h; Mannich reaction; Neat (no solvent); | 92% |

-
-
109-12-6
2-aminopyrimidine

-
-
135-02-4
ortho-anisaldehyde

-
-
135-19-3
β-naphthol

-
-
1190380-97-2
1-[2-methoxyphenyl((pyrimidin-2-yl)amino)methyl]naphthalen-2-ol

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In 1,2-dichloro-ethane for 3h; Reflux; | 97% |

2-Aminopyrimidine Consensus Reports
2-Aminopyrimidine Specification
The 2-Aminopyrimidine, with the CAS registry number 109-12-6 and EINECS registry number 203-648-4, has the systematic name of pyrimidin-2-amine. And the molecular formula of this chemical is C4H5N3. It is a kind of white to light yellow powder, and belongs to the following product categories: Pyrimidine; Fine chemical & intermediates; APIs & Intermediate; Amines; Nucleic acids.
Physical properties about 2-Aminopyrimidine are: (1)ACD/LogP: -0.22; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -0.23; (4)ACD/LogD (pH 7.4): -0.22; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 17.68; (8)ACD/KOC (pH 7.4): 18.08; (9)#H bond acceptors: 3; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 29.02 Å2; (13)Index of Refraction: 1.598; (14)Molar Refractivity: 26.67 cm3; (15)Molar Volume: 78.1 cm3; (16)Polarizability: 10.57×10-24cm3; (17)Surface Tension: 64.3 dyne/cm; (18)Density: 1.216 g/cm3; (19)Flash Point: 144.5 °C; (20)Enthalpy of Vaporization: 51.35 kJ/mol; (21)Boiling Point: 275.1 °C at 760 mmHg; (22)Vapour Pressure: 0.00521 mmHg at 25°C.
Preparation and uses of 2-Aminopyrimidine: It can be prepared by from Mucochloric acid and guanidine nitrate via cyclization, decarboxylation and dechlorination. What's more, it is often used in the biochemical study and organic systhesis.
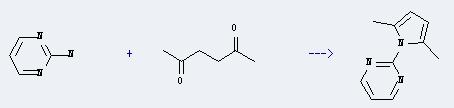
Chemical reaction: It is used in the synthesis of other chemicals. For example, it can react with hexane-2,5-dione to produce 2-(2,5-dimethyl-pyrrol-1-yl)-pyrimidine. The reaction time is 24 hours with temperature of 100°C, and the yield is about 36%.
You should be cautious while dealing with this chemical. It irritates eyes, respiratory system and skin. Therefore, you had better take the following instructions: Wear suitable protective clothing, gloves and eye/face protection, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: c1cnc(nc1)N
(2)InChI: InChI=1/C4H5N3/c5-4-6-2-1-3-7-4/h1-3H,(H2,5,6,7)
(3)InChIKey: LJXQPZWIHJMPQQ-UHFFFAOYAJ
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | intravenous | 790mg/kg (790mg/kg) | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 211, Pg. 367, 1950. |
Related Products
- 2-Aminopyrimidine
- 2-Aminopyrimidine-4-carboxylic acid
- 2-Aminopyrimidine-5-boronic acid
- 2-Aminopyrimidine-5-boronic acid pinacol ester
- 2-Aminopyrimidine-5-carbonitrile
- 2-Aminopyrimidine-5-carboxylic acid
- 109-13-7
- 109140-20-7
- 109142-53-2
- 109151-82-8
- 109152-85-4
- 109-15-9
- 109-16-0
- 1091605-42-3
- 109170-71-0
- 109-17-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View