-
Name
2-Chloropyrazine
- EINECS 238-517-0
- CAS No. 14508-49-7
- Article Data25
- CAS DataBase
- Density 1.303 g/cm3
- Solubility insoluble in water
- Melting Point 153~154℃
- Formula C4H3ClN2
- Boiling Point 155.3 °C at 760 mmHg
- Molecular Weight 114.534
- Flash Point 57 °C
- Transport Information UN 1993 3/PG 3
- Appearance clear colorless to yellowish liquid
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Pyrazine,chloro- (6CI,7CI,8CI,9CI);Pyrazine,2-chloro-;Chloropyrazine;Pyrazin-2-ylchloride;
- PSA 25.78000
- LogP 1.13000
Synthetic route
-
-
24387-68-6
2-hydroxypyrazine sodium salt

-
-
14508-49-7
2-chloropyrazin

| Conditions | Yield |
|---|---|
| With thionyl chloride; N-ethyl-N,N-diisopropylamine In chloroform at 65℃; for 2h; Reagent/catalyst; | 95.4% |
-
-
6270-63-9
pyrazin-2-ol

-
-
14508-49-7
2-chloropyrazin

| Conditions | Yield |
|---|---|
| With dmap; thionyl chloride; bis(trichloromethyl) carbonate for 10.5h; Reflux; Green chemistry; | 89% |
| With trichlorophosphate for 0.666667h; Heating; | 84% |
| With trichlorophosphate |
-
-
98-97-5
2-pyrazylcarboxylic acid

-
-
14508-49-7
2-chloropyrazin

| Conditions | Yield |
|---|---|
| With thionyl chloride In pyridine; para-tert-butylphenol; benzene | 75% |
-
-
2423-65-6
Pyrazin-N-Oxid(PyzNO)

-
-
14508-49-7
2-chloropyrazin

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 70℃; for 2h; | 37% |
| With trichlorophosphate | 35% |
-
-
2423-65-6
Pyrazin-N-Oxid(PyzNO)

-
A
-
14508-49-7
2-chloropyrazin

| Conditions | Yield |
|---|---|
| With trichlorophosphate for 0.5h; Product distribution; Ambient temperature; | A 13% B 30% C n/a |

-
-
290-37-9
1,4-pyrazine

-
-
14508-49-7
2-chloropyrazin

| Conditions | Yield |
|---|---|
| With chlorine at 500℃; | |
| With water; chlorine at 450℃; |
-
-
6270-63-9
pyrazin-2(1H)-one

-
-
14508-49-7
2-chloropyrazin

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride; trichlorophosphate | |
| With trichlorophosphate |
-
-
288-32-4
1H-imidazole

-
-
67-66-3
chloroform

-
A
-
14508-49-7
2-chloropyrazin

-
B
-
17180-94-8
5-chloropyrimidine

| Conditions | Yield |
|---|---|
| at 550℃; Yield given. Yields of byproduct given; |

-
-
616-47-7
1-methyl-1H-imidazole

-
-
67-66-3
chloroform

-
A
-
14508-49-7
2-chloropyrazin

-
B
-
4513-94-4
1H-pyrrole-2-carbonitrile

-
C
-
17180-94-8
5-chloropyrimidine

-
D
-
51269-82-0
5-chloronicotinonitrile

| Conditions | Yield |
|---|---|
| at 550℃; Yield given. Further byproducts given. Yields of byproduct given; |

-
-
616-47-7
1-methyl-1H-imidazole

-
-
67-66-3
chloroform

-
A
-
14508-49-7
2-chloropyrazin

-
B
-
17180-94-8
5-chloropyrimidine

-
C
-
89809-64-3
2-cyano-5-chloropyridine

-
D
-
51269-82-0
5-chloronicotinonitrile

| Conditions | Yield |
|---|---|
| at 550℃; Yield given. Further byproducts given. Yields of byproduct given; |

-
-
616-47-7
1-methyl-1H-imidazole

-
-
67-66-3
chloroform

-
A
-
14508-49-7
2-chloropyrazin

-
B
-
17180-94-8
5-chloropyrimidine

-
C
-
51269-82-0
5-chloronicotinonitrile

-
D
-
38180-46-0
3-chloro-2-cyanopyridine

| Conditions | Yield |
|---|---|
| at 550℃; Yield given. Further byproducts given. Yields of byproduct given; |

-
-
616-47-7
1-methyl-1H-imidazole

-
-
67-66-3
chloroform

-
A
-
14508-49-7
2-chloropyrazin

-
B
-
17180-94-8
5-chloropyrimidine

-
C
-
51269-82-0
5-chloronicotinonitrile

-
D
-
68325-15-5
3-chloro-isonicotinonitrile

| Conditions | Yield |
|---|---|
| at 550℃; Yield given. Further byproducts given. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With sodium hydroxide; trichlorophosphate 1) 70 deg C, 2 h; Yield given. Multistep reaction. Yields of byproduct given; |

| Conditions | Yield |
|---|---|
| With trichlorophosphate at 70℃; for 2h; Product distribution; | A 50 % Spectr. B 50 % Spectr. |
-
-
67-66-3
chloroform

-
-
18156-74-6
1-(Trimethylsilyl)imidazole

-
A
-
14508-49-7
2-chloropyrazin

-
B
-
17180-94-8
5-chloropyrimidine

| Conditions | Yield |
|---|---|
| at 550℃; Yield given. Yields of byproduct given; |

-
-
6863-76-9
3-chloropyrazine 1-oxide

-
A
-
14508-49-7
2-chloropyrazin

-
B
-
19745-07-4
2,5-dichloropyrazine

-
C
-
4858-85-9
2,3-dichloropyrazine

-
D
-
4774-14-5
2,6-dichloropyrazine

| Conditions | Yield |
|---|---|
| With trichlorophosphate for 2h; Heating; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
16025-16-4
2-chloropyrazine 1-oxide

-
A
-
14508-49-7
2-chloropyrazin

-
B
-
4858-85-9
2,3-dichloropyrazine

-
C
-
4774-14-5
2,6-dichloropyrazine

| Conditions | Yield |
|---|---|
| With trichlorophosphate for 2h; Heating; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
290-37-9
1,4-pyrazine

-
-
7732-18-5
water

-
-
7782-50-5
chlorine

-
A
-
14508-49-7
2-chloropyrazin

-
B
-
19745-07-4
2,5-dichloropyrazine

-
C
-
4858-85-9
2,3-dichloropyrazine

-
D
-
4774-14-5
2,6-dichloropyrazine

| Conditions | Yield |
|---|---|
| at 350℃; Product distribution; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) heating in Ar at 220 °C; detected by powder X-ray diffractometry; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) in air, heating at 180 °C, CuCl, 2-chloropyrazine and some CuO were formed, Cu2OCl2 was formed at 392 °C, CuCl was formed again at430 °,C and CuO was formed in the last step at 461 °C; detected by X-ray powder diffractometry; |

-
-
419581-32-1
CuBr(μ-2-chloropyrazine-N,N')

-
A
-
14508-49-7
2-chloropyrazin

-
B
-
7787-70-4
copper(I) bromide

| Conditions | Yield |
|---|---|
| In neat (no solvent) heating in Ar at 200 °C; detected by powder X-ray diffractometry; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) in air, CuBr and 2-chloropyrazine were formed at 171 °C, CuBr wasoxidised during further heating; detected by X-ray powder diffractometry; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) heating in Ar at 150 °C; detected by powder X-ray diffractometry; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) in air, heating at 138 °C, Cu2I2(2-chloropyrazine) then CuI and 2-chloropyrazine were formed, CuO was formed in the last step around 340 °C; detected by X-ray powder diffractometry; |


| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride; tricyclohexylphosphine; dichloro bis(acetonitrile) palladium(II) at 85 - 90℃; for 5h; Suzuki-Miyaura reaction; | 100% |
| With potassium phosphate; C46H52Cl3N3O2Pd In tetrahydrofuran; water at 60℃; for 4h; Suzuki-Miyaura Coupling; | 99% |
| With 2-((3-sulfonatomesityl)-5-sulfonatoindenyl)dicyclohexylphosphinehydrate sodium salt; palladium diacetate; potassium carbonate In water at 100℃; for 24h; Suzuki-Miyaura Coupling; | 95% |
-
-
14508-49-7
2-chloropyrazin

-
-
38521-06-1
2-mercaptopyrazine

| Conditions | Yield |
|---|---|
| With thiourea In ethanol for 16h; Reflux; | 100% |
| With hydrogen sulfide; sodium methylate In methanol for 1h; Heating; | 91% |
| With hydrogen sulfide; sodium ethanolate; N,N-dimethyl-formamide In ethanol at 100℃; for 3h; | 88% |

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); ruphos In tetrahydrofuran at 20℃; for 15h; Negishi coupling; | 100% |

| Conditions | Yield |
|---|---|
| at 100℃; for 15h; | 100% |
| In ethanol at 95℃; for 1h; Inert atmosphere; Sealed tube; | 77% |
| In dimethyl sulfoxide at 130℃; |
-
-
14508-49-7
2-chloropyrazin

-
-
109384-19-2
t-butyl 4-hydroxy piperidine-1-carboxylate

-
-
442199-08-8
t-butyl 4-[(pyrazin-2-yl)oxy]piperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; for 2h; | 100% |
| In hexane; dimethyl sulfoxide; ethyl acetate |
-
-
14508-49-7
2-chloropyrazin

-
-
21450-64-6
(2,6-dimethylphenyl)magnesium bromide

-
-
1157867-58-7
2-(2,6-dimethylphenyl)pyrazine

| Conditions | Yield |
|---|---|
| Stage #1: (2,6-dimethylphenyl)magnesium bromide With dichloro(1,3-bis(2,6-bis(3-pentyl)phenyl)imidazolin-2-ylidene)(3-chloropyridyl)palladium(II); zinc(II) chloride In tetrahydrofuran at 23℃; for 0.333333h; Inert atmosphere; Stage #2: 2-chloropyrazin In tetrahydrofuran; 1-methyl-pyrrolidin-2-one at 23℃; for 24h; Negishi coupling; Inert atmosphere; | 100% |
-
-
14508-49-7
2-chloropyrazin

-
-
110-70-3
N,N`-dimethylethylenediamine

| Conditions | Yield |
|---|---|
| In ethanol | 100% |
-
-
14508-49-7
2-chloropyrazin

-
-
67-56-1
methanol

-
-
201230-82-2
carbon monoxide

-
-
6164-79-0
methyl pyrazine-2-carboxylate

| Conditions | Yield |
|---|---|
| With dichloro[2,2'-bis(diphenylphosphino)-1,1'-binaphthyl]palladium(II); triethylamine at 100℃; under 2585.74 Torr; for 4h; | 99% |
| With 1,3-bis-(diphenylphosphino)propane; palladium diacetate; triethylamine In acetonitrile at 180℃; under 25502.6 Torr; Flow reactor; | 61% |
| With triethylamine; palladium bis(dibenzylideneacetone)palladium(0); triphenylphosphine at 120℃; under 29420.3 Torr; for 16h; other chloropyrazines; | 93 % Chromat. |
| With ethyl azide; triphenylphosphine; bis(dibenzylideneacetone)-palladium(0) at 120℃; under 29420.3 Torr; for 16h; | 93 % Chromat. |


| Conditions | Yield |
|---|---|
| With potassium phosphate; bis[dicyclohexyl(2,4,6-triisopropyl-[1,1':3',1''-terphenyl]-2-yl)phosphane]palladium(II) dichloride In acetonitrile at 90℃; for 6h; Sonogashira Cross-Coupling; | 99% |
| With tetrabutylammomium bromide; potassium carbonate; triphenylphosphine; copper(I) oxide at 135 - 140℃; for 24h; Sonogashira coupling; | 98% |
| With bis(η3-allyl-μ-chloropalladium(II)); triethylamine; triphenylphosphine; 1-butyl-3-methylimidazolium Tetrafluoroborate at 100℃; for 4h; Heck alkynylation; Inert atmosphere; | 95% |
| With dichloro bis(acetonitrile) palladium(II); 2-((3-sulfonatomesityl)-5-sulfonatoindenyl)dicyclohexylphosphinehydrate sodium salt; caesium carbonate In water; acetonitrile at 100℃; for 24h; Sonogashira Cross-Coupling; | 89% |
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; diisopropylamine at 70℃; for 12h; Sonogashira coupling; | 76% |
-
-
14508-49-7
2-chloropyrazin

-
-
100379-00-8
2,6-dimethylbenzene boronic acid

-
-
1157867-58-7
2-(2,6-dimethylphenyl)pyrazine

| Conditions | Yield |
|---|---|
| With trans-dichloro(1,3-bis-(2,6-diisopentylphenyl)imidazolylidinium)(3-chloro-pyridine); potassium tert-butylate In tert-butyl alcohol at 65℃; Suzuki-Miyaura coupling; Inert atmosphere; Molecular sieve; | 99% |
-
-
14508-49-7
2-chloropyrazin

-
-
5720-05-8
4-methylphenylboronic acid

-
-
108030-80-4
2-(4-methylphenyl)pyrazine

| Conditions | Yield |
|---|---|
| With potassium phosphate tribasic heptahydrate; palladium diacetate In ethylene glycol at 80℃; for 0.333333h; Suzuki coupling; | 99% |
| With (1,3-bis(2,6-diisopropylphenyl)-3,4,5,6-tetrahydropyrimidin-2-ylidene)Pd(cinnamyl, 3-phenylallyl)Cl; tetrabutylammomium bromide; sodium hydrogencarbonate In water for 1h; Catalytic behavior; Suzuki-Miyaura Coupling; Reflux; | 98% |
| Stage #1: 2-chloropyrazin; 4-methylphenylboronic acid With tetrakis(triphenylphosphine) palladium(0) In water; toluene at 95℃; for 0.166667h; Suzuki Coupling; Inert atmosphere; Stage #2: With potassium fluoride dihydrate In water; toluene at 95℃; Suzuki Coupling; Inert atmosphere; | 96% |
| With potassium phosphate tribasic heptahydrate; palladium dichloride In water; N,N-dimethyl-formamide at 20℃; for 24h; Suzuki cross-coupling reaction; | 88% |
| With potassium phosphate tribasic heptahydrate; palladium diacetate In isopropyl alcohol at 80℃; for 0.666667h; Suzuki coupling; | 82% |

-
-
14508-49-7
2-chloropyrazin

-
-
149775-45-1
6-Methyl-2-(2-pyrazinyl)pyridine

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); XPhos In tetrahydrofuran at 65℃; for 3h; Negishi cross-coupling; Inert atmosphere; | 99% |
-
-
14508-49-7
2-chloropyrazin

-
-
5720-06-9
2-Methoxyphenylboronic acid

-
-
251933-26-3
2-(2-methoxyphenyl)pyrazine

| Conditions | Yield |
|---|---|
| With (4-(N,N-dimethylamino)phenyl)-di-tert-butylphosphine; palladium diacetate; potassium carbonate In ethanol; water at 80℃; for 3h; Catalytic behavior; Reagent/catalyst; Suzuki-Miyaura Coupling; Inert atmosphere; | 99% |
| With 2-((3-sulfonatomesityl)-5-sulfonatoindenyl)dicyclohexylphosphinehydrate sodium salt; palladium diacetate; potassium carbonate In water at 100℃; for 24h; Suzuki-Miyaura Coupling; | 91% |
-
-
14508-49-7
2-chloropyrazin

-
-
10365-98-7
3-methoxyphenylboronic acid

-
-
912771-38-1
2-(3-methoxyphenyl)-pyrazine

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloropyrazin; 3-methoxyphenylboronic acid With tetrakis(triphenylphosphine) palladium(0) In water; toluene at 95℃; for 0.166667h; Suzuki Coupling; Inert atmosphere; Stage #2: With potassium fluoride dihydrate In water; toluene at 95℃; Suzuki Coupling; Inert atmosphere; | 99% |
-
-
14508-49-7
2-chloropyrazin

-
-
139911-29-8
4-fluoro-2-methylphenylboronic acid

-
-
334476-87-8
2-(4-fluoro-2-methylphenyl)pyrazine

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloropyrazin; 4-fluoro-2-methylphenylboronic acid With tetrakis(triphenylphosphine) palladium(0) In water; toluene at 95℃; for 0.166667h; Suzuki Coupling; Inert atmosphere; Stage #2: With potassium fluoride dihydrate In water; toluene at 95℃; Suzuki Coupling; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-Methoxybenzyl alcohol With sodium hydride In 1,2-dimethoxyethane at 0℃; for 0.333333h; Inert atmosphere; Stage #2: 2-chloropyrazin In 1,2-dimethoxyethane at 0 - 45℃; Inert atmosphere; | 99% |
-
-
14508-49-7
2-chloropyrazin

-
-
333-20-0
potassium thioacyanate

| Conditions | Yield |
|---|---|
| In water at 20℃; for 24h; | 98.6% |

-
-
14508-49-7
2-chloropyrazin

-
-
13689-92-4
nickel(II) thiocyanate

| Conditions | Yield |
|---|---|
| at 20℃; for 72h; | 98.5% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 168h; | 98.3% |
-
-
14508-49-7
2-chloropyrazin

-
-
128190-66-9, 25088-58-8
potassium enolate of acetone

-
-
6784-62-9
1-(pyrazin-2-yl)propan-2-one

| Conditions | Yield |
|---|---|
| In diethyl ether; ammonia for 0.25h; Mechanism; Product distribution; effect of inhibitor (di-tert-butyl-nitroxide); | 98% |

| Conditions | Yield |
|---|---|
| tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran for 36h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| With C84H64Cl3N3Pd; potassium carbonate In ethanol at 80℃; for 2h; Suzuki-Miyaura Coupling; | 98% |
| With tetrabutyl ammonium fluoride; tricyclohexylphosphine; dichloro bis(acetonitrile) palladium(II) at 85 - 90℃; for 5h; Suzuki-Miyaura reaction; | 95% |
| With 2-((3-sulfonatomesityl)-5-sulfonatoindenyl)dicyclohexylphosphinehydrate sodium salt; palladium diacetate; potassium carbonate In water at 100℃; for 24h; Suzuki-Miyaura Coupling; | 87% |
| With potassium phosphate; tetrakis(triphenylphosphine) palladium(0) In 1,4-dioxane at 160℃; for 0.5h; Suzuki Coupling; Microwave irradiation; | 71% |
| With 1,4-diaza-bicyclo[2.2.2]octane; potassium hydroxide; palladium diacetate In N,N-dimethyl-formamide at 110℃; for 38h; Suzuki-Miyaura cross-coupling; | 50% |

| Conditions | Yield |
|---|---|
| With trans-dichloro(1,3-bis-(2,6-diisopentylphenyl)imidazolylidinium)(3-chloro-pyridine); cesium fluoride In 1,4-dioxane at 20 - 80℃; for 32h; Stille-Migita cross-coupling; Molecular sieve; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloropyrazin; 4-fluoroboronic acid With tetrakis(triphenylphosphine) palladium(0) In water; toluene at 95℃; for 0.166667h; Suzuki Coupling; Inert atmosphere; Stage #2: With potassium fluoride dihydrate In water; toluene at 95℃; Suzuki Coupling; Inert atmosphere; | 98% |
| With palladium diacetate; potassium carbonate In ethanol; water at 80℃; for 0.5h; Suzuki Coupling; | 90% |
| With potassium phosphate tribasic heptahydrate; palladium diacetate In ethylene glycol at 80℃; for 1.58333h; Suzuki coupling; | 88% |
-
-
14508-49-7
2-chloropyrazin

-
-
16419-60-6
2-Methylphenylboronic acid

-
-
87537-41-5
2-(2-methylphenyl)pyrazine

| Conditions | Yield |
|---|---|
| With C84H64Cl3N3Pd; potassium carbonate In ethanol at 80℃; for 2h; Suzuki-Miyaura Coupling; | 98% |
| With 2-((3-sulfonatomesityl)-5-sulfonatoindenyl)dicyclohexylphosphinehydrate sodium salt; palladium diacetate; potassium carbonate In water at 100℃; for 24h; Suzuki-Miyaura Coupling; | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloropyrazin; m-tolylboronic acid With tetrakis(triphenylphosphine) palladium(0) In water; toluene at 95℃; for 0.166667h; Suzuki Coupling; Inert atmosphere; Stage #2: With potassium fluoride dihydrate In water; toluene at 95℃; Suzuki Coupling; Inert atmosphere; | 98% |
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In ethanol at 80℃; for 2.5h; Inert atmosphere; | 95% |
| With potassium phosphate tribasic trihydrate; C30H39P; palladium diacetate In tetrahydrofuran at 110℃; for 3h; Suzuki-Miyaura Coupling; Inert atmosphere; | 93% |
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In ethanol at 80℃; for 2.5h; |

| Conditions | Yield |
|---|---|
| With C84H64Cl3N3Pd; potassium carbonate In ethanol at 80℃; for 2h; Suzuki-Miyaura Coupling; | 98% |
| With potassium phosphate; C46H52Cl3N3O2Pd In tetrahydrofuran; water at 60℃; for 4h; Suzuki-Miyaura Coupling; | 91% |

| Conditions | Yield |
|---|---|
| In water at 20℃; for 48h; | 97.6% |

| Conditions | Yield |
|---|---|
| With bis(η3-allyl-μ-chloropalladium(II)); 1,1',3,3'-tetrakis(diphenylphosphino)ferrocene; sodium t-butanolate In toluene for 17h; Catalytic behavior; Inert atmosphere; Schlenk technique; Heating; | 97% |
| at 28℃; for 12h; | 94% |
| Stage #1: 2-chloropyrazin With (1,3,5-triaza-7-phosphaadamantan-1-ium-1-yl)butane-1-sulfonate; palladium diacetate In N,N-dimethyl-formamide for 0.0833333h; Schlenk technique; Inert atmosphere; Stage #2: thiophenol With potassium phosphate In N,N-dimethyl-formamide at 50℃; for 2h; Catalytic behavior; Temperature; Solvent; Reagent/catalyst; Schlenk technique; Inert atmosphere; | 88% |
| With 1,1'-bis-(diphenylphosphino)ferrocene; bis(η3-allyl-μ-chloropalladium(II)); sodium t-butanolate In toluene at 120℃; for 17h; | 86% |
| With sodium hydride In dimethyl sulfoxide for 5h; Heating; | 70% |
-
-
14508-49-7
2-chloropyrazin

-
-
10199-00-5
2,2'-bipyrazine

| Conditions | Yield |
|---|---|
| With palladium diacetate; tetrabutylammomium bromide; N-ethyl-N,N-diisopropylamine In isopropyl alcohol; toluene at 105℃; for 24h; | 97% |
| With potassium hydroxide In water; N,N-dimethyl-formamide at 35℃; for 12h; Ullmann Condensation; Inert atmosphere; | 94% |
| With nickel diacetate; triphenylphosphine; sodium t-butanolate In 1,2-dimethoxyethane at 45℃; for 0.00277778h; | 48% |
| With tetrakis(triphenylphosphine) palladium(0); tetrabutylammomium bromide; potassium carbonate In N,N-dimethyl-formamide at 140℃; for 16h; | 42% |
| With palladium diacetate; potassium carbonate In isopropyl alcohol; toluene at 100℃; for 5h; Reagent/catalyst; Inert atmosphere; | 3% |
2-Chloropyrazine Chemical Properties
Molecular Structure of 2-Chloropyrazine (CAS NO.14508-49-7):
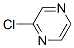
IUPAC Name: 2-Chloropyrazine
Molecular Formula: C4H3ClN2
Molecular Weight: 114.53 g/mol
Density: 1.303 g/cm3
Flash Point: 57.8 °C
Boiling Point: 155.3 °C at 760 mmHg
Water Solubility: insoluble
Index of Refraction: 1.534
Molar Refractivity: 27.33 cm3
Molar Volume: 87.8 cm3
Surface Tension: 48.2 dyne/cm
XLogP3: 0.7
H-Bond Acceptor: 2
Exact Mass: 113.998476
MonoIsotopic Mass: 113.998476
Topological Polar Surface Area: 25.8
Heavy Atom Count: 7
Canonical SMILES: C1=CN=C(C=N1)Cl
InChI: InChI=1S/C4H3ClN2/c5-4-3-6-1-2-7-4/h1-3H
InChIKey: GELVZYOEQVJIRR-UHFFFAOYSA-N
EINECS: 238-517-0
Product Categories: Halides; Heterocycles; Pyrazines; Pyrazine; Chloropyrazines, etc.; Building Blocks; Halogenated Heterocycles; Heterocyclic Building Blocks; PyrazinesHeterocyclic Building Blocks
2-Chloropyrazine Uses
2-Chloropyrazine (CAS NO.14508-49-7) is used as intermediates of medicine and pesticide.
2-Chloropyrazine Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36-37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S37/39:Wear suitable gloves and eye/face protection.
RIDADR: UN 1993 3/PG 3
WGK Germany: 3
Hazard Note: Irritant
TSCA: T
HazardClass: 3
PackingGroup: III
HS Code: 29339990
2-Chloropyrazine Specification
2-Chloropyrazine (CAS NO.14508-49-7) is also named as Chloropyrazine ; Pyrazine, 2-chloro- ; Pyrazine, chloro- . 2-Chloropyrazine (CAS NO.14508-49-7) is clear colorless to yellowish liquid.
Related Products
- 2-Chloropyrazine
- 1450-85-7
- 1450-93-7
- 1450-95-9
- 145099-21-4
- 145099-40-7
- 145100-50-1
- 145100-51-2
- 14510-06-6
- 14510-37-3
- 145104-99-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View