-
Name
2-Ethylaniline
- EINECS 209-424-2
- CAS No. 578-54-1
- Article Data100
- CAS DataBase
- Density 0.973 g/cm3
- Solubility insoluble in water
- Melting Point -44 °C
- Formula C8H11N
- Boiling Point 212.3 °C at 760 mmHg
- Molecular Weight 121.182
- Flash Point 87.9 °C
- Transport Information UN 2273 6.1/PG 3
- Appearance clear yellow to red-brownish liquid
- Safety 36/37/39-45
- Risk Codes 36/37/38-33-23/24/25
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms Aniline,o-ethyl- (8CI);2-Ethylaniline;2-Ethylbenzenamine;2-Ethylphenylamine;NSC62014;o-Aminoethylbenzene;o-Ethylaniline;
- PSA 26.02000
- LogP 2.41240
Synthetic route

| Conditions | Yield |
|---|---|
| With dichloro(dimethylglyoxime)(dimethylglyoximato)cobalt(III); (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate; triethylamine In acetonitrile at -78℃; for 24h; Reagent/catalyst; Sealed tube; Inert atmosphere; Irradiation; | 100% |

| Conditions | Yield |
|---|---|
| With hydrogen In methanol at 20℃; under 750.075 Torr; for 1h; chemoselective reaction; | 99.6% |
| With hydrogen; Pd in AV-17-8-Pd In ethanol at 40℃; | 98% |
| With hydrogen; Pd in AV-17-8-Pd In ethanol at 40℃; under 760 Torr; Rate constant; | 98% |
-
-
35774-47-1
1-azido-2-ethylbenzene

-
-
578-54-1
ortho-ethylaniline

| Conditions | Yield |
|---|---|
| With iron In water at 20℃; Inert atmosphere; | 90% |
-
-
612-22-6
2-nitro(ethylbenzene)

-
A
-
578-54-1
ortho-ethylaniline

-
B
-
64287-80-5
Bis(2-ethylphenyl)diazene 1-oxide

| Conditions | Yield |
|---|---|
| With ammonium chloride; zinc In water at 80℃; for 24h; Catalytic behavior; Inert atmosphere; | A 8% B 84% |

| Conditions | Yield |
|---|---|
| In gaseous matrix at 80℃; Irradiation; | A 82% B 18 % Spectr. |
| In gaseous matrix at 80℃; Irradiation; | A 82 % Spectr. B n/a |

-
-
155690-08-7
2-p-Tolyl-3H,4H-2λ4-benzo[c][1,2]thiazine 2-oxide

-
-
578-54-1
ortho-ethylaniline

| Conditions | Yield |
|---|---|
| With disodium hydrogenphosphate; sodium amalgam In tetrahydrofuran; methanol for 18h; Ambient temperature; | 81% |

| Conditions | Yield |
|---|---|
| With ammonium sulfate; bis(1,5-cyclooctadiene)nickel (0); sodium t-butanolate at 100 - 110℃; for 12h; | 79% |

| Conditions | Yield |
|---|---|
| With ammonium sulfate; (R)-(-)-1-[(SP)-2-(dicyclohexylphosphino)ferrocenyl]ethyldi-tert-butylphosphine; bis(tri-ortho-tolylphosphine)palladium(0); sodium t-butanolate In 1,4-dioxane at 100℃; for 8h; Inert atmosphere; Glovebox; | 76% |
-
-
52670-38-9
2-ethynylaniline

-
-
578-54-1
ortho-ethylaniline

| Conditions | Yield |
|---|---|
| With [Fe(nacnac)dippCH2SiMe3]; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane In benzene-d6 at 20℃; for 0.5h; Sealed tube; Inert atmosphere; Schlenk technique; Glovebox; | 74% |
| With palladium diacetate; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane In dichloromethane at 25℃; for 12h; Sealed tube; Inert atmosphere; chemoselective reaction; | 42% |
-
-
109-72-8, 29786-93-4
n-butyllithium

-
-
35774-47-1
1-azido-2-ethylbenzene

-
A
-
578-54-1
ortho-ethylaniline

-
B
-
204926-79-4
N-butyl-2-ethylphenylamine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -78 - 20℃; for 3h; | A 69% B 8% |

-
-
848170-57-0
2-ethylphenyl tosylate

-
-
578-54-1
ortho-ethylaniline

| Conditions | Yield |
|---|---|
| Stage #1: 2-ethylphenyl tosylate With [(k2-P,N-di(1-adamantyl)-2-morpholinophenylphosphine)Pd(Ph)Cl]; sodium t-butanolate In 1,4-dioxane Inert atmosphere; Glovebox; Stage #2: With ammonia In 1,4-dioxane at 24℃; Inert atmosphere; Glovebox; | 66% |
-
-
635-46-1
1,2,3,4-tetrahydroisoquinoline

-
-
612-22-6
2-nitro(ethylbenzene)

-
A
-
91-22-5
quinoline

-
B
-
578-54-1
ortho-ethylaniline

| Conditions | Yield |
|---|---|
| With nickel-nitrogen-doped carbon framework In water at 145℃; for 18h; Inert atmosphere; Sealed tube; Green chemistry; | A 58% B 62% |


| Conditions | Yield |
|---|---|
| In gaseous matrix at 80℃; Irradiation; | A 57% B 43 % Spectr. |

-
-
153856-89-4
7-amino-1,2,3,4-tetrahydroquinoline

-
-
612-22-6
2-nitro(ethylbenzene)

-
A
-
580-19-8
quinolin-7-ylamine

-
B
-
578-54-1
ortho-ethylaniline

| Conditions | Yield |
|---|---|
| With nickel-nitrogen-doped carbon framework In water at 145℃; for 18h; Inert atmosphere; Sealed tube; Green chemistry; | A 51% B 54% |

-
-
120-15-0
6-methoxy-1,2,3,4-tetrahydroquinoline

-
-
612-22-6
2-nitro(ethylbenzene)

-
A
-
5263-87-6
6-methoxy quinoline

-
B
-
578-54-1
ortho-ethylaniline

| Conditions | Yield |
|---|---|
| With nickel-nitrogen-doped carbon framework In water at 145℃; for 18h; Inert atmosphere; Sealed tube; Green chemistry; | A 48% B 52% |


| Conditions | Yield |
|---|---|
| With formic acid; triethylamine; palladium on activated charcoal at 100℃; | 51% |
| Stage #1: 2-acetylnitrobenzene With hydrazine hydrate at 135℃; for 3h; Stage #2: With potassium hydroxide at 135℃; for 24h; | 33% |
-
-
100-41-4
ethylbenzene

-
A
-
578-54-1
ortho-ethylaniline

-
B
-
587-02-0
m-ethylaniline

-
C
-
589-16-2
4-aminoethylbenzene

| Conditions | Yield |
|---|---|
| With tris-(2-chloro-ethyl)-amine; trifluorormethanesulfonic acid; trifluoroacetic acid In chloroform at 25℃; for 4h; | A 47% B 8% C 34% |
| With 1-aminoquinolinium perchlorate In trifluoroacetic acid for 4h; Irradiation; | A 29% B 20% C 32% |
| With 18-crown-6 ether; 1-aminoquinolinium perchlorate In trifluoroacetic acid for 4h; Irradiation; | A 29% B 22% C 31% |

-
-
120-72-9
indole

-
A
-
578-54-1
ortho-ethylaniline

-
B
-
1678-91-7
ethyl-cyclohexane

-
C
-
100-41-4
ethylbenzene

-
D
-
95-53-4
o-toluidine

| Conditions | Yield |
|---|---|
| With hydrogen sulfide; hydrogen; MoOxNy at 326.85℃; under 68255.5 Torr; Product distribution; Further Variations:; Catalysts; hydrodenitrogenation; | A 45.2% B 15.9% C 6.2% D 9.3% |

-
-
612-22-6
2-nitro(ethylbenzene)

-
A
-
578-54-1
ortho-ethylaniline

-
B
-
16433-80-0
1-ethyl-2-nitrosobenzene

-
C
-
64287-80-5
Bis(2-ethylphenyl)diazene 1-oxide

| Conditions | Yield |
|---|---|
| With ammonium chloride; zinc In water at 80℃; for 3.33333h; Catalytic behavior; Inert atmosphere; | A 45% B 24% C 19% |

-
-
14936-94-8
ethyl cation

-
-
62-53-3
aniline

-
A
-
578-54-1
ortho-ethylaniline

-
B
-
589-16-2
4-aminoethylbenzene

| Conditions | Yield |
|---|---|
| Product distribution; reactions with other charged electrophiles; | A 44% B 34% C n/a |

-
-
34557-54-5
methane

-
-
62-53-3
aniline

-
A
-
578-54-1
ortho-ethylaniline

-
B
-
587-02-0
m-ethylaniline

-
C
-
589-16-2
4-aminoethylbenzene

| Conditions | Yield |
|---|---|
| In gaseous matrix at 80℃; Irradiation; | A 63 % Spectr. B 2% C 35% |
| With oxygen; trimethylamine at 80℃; Product distribution; Irradiation; |

-
-
612-22-6
2-nitro(ethylbenzene)

-
A
-
578-54-1
ortho-ethylaniline

-
B
-
16433-80-0
1-ethyl-2-nitrosobenzene

-
C
-
61653-34-7
1,2-bis(2-ethylphenyl)diazene

-
D
-
64287-80-5
Bis(2-ethylphenyl)diazene 1-oxide

| Conditions | Yield |
|---|---|
| With ammonium chloride; zinc In water at 80℃; for 3.33333h; Catalytic behavior; Inert atmosphere; | A 32% B 32% C 7% D 24% |

-
-
77-77-0
Divinyl sulfone

-
-
612-22-6
2-nitro(ethylbenzene)

-
A
-
578-54-1
ortho-ethylaniline

-
B
-
1219600-77-7
4-(2-ethylphenyl)-thiomorpholine 1,1-dioxide

| Conditions | Yield |
|---|---|
| With indium; acetic acid In methanol for 24h; aza-Michael type 1,4-addition reaction; Inert atmosphere; Reflux; | A 24% B 12% |

-
-
97-93-8
triethylaluminum

-
-
98-95-3
nitrobenzene

-
A
-
578-54-1
ortho-ethylaniline

-
B
-
103-69-5
N-ethyl-N-phenylamine

-
C
-
62-53-3
aniline

-
D
-
589-16-2
4-aminoethylbenzene

| Conditions | Yield |
|---|---|
| In hexane for 0.5h; Ambient temperature; Further byproducts given; | A 9.1% B 13.7% C 15.4% D 5% |


| Conditions | Yield |
|---|---|
| With nickel; decalin at 225℃; under 19000 Torr; Hydrogenation; |

| Conditions | Yield |
|---|---|
| With nickel; decalin at 225℃; under 19000 Torr; Hydrogenation; |
-
-
623-81-4
diethyl sulphite

-
-
62-53-3
aniline

-
A
-
578-54-1
ortho-ethylaniline

-
B
-
91-66-7
N,N-diethylaniline


| Conditions | Yield |
|---|---|
| With aniline; aluminium anilide at 300 - 350℃; under 110326 - 147102 Torr; |

| Conditions | Yield |
|---|---|
| With aluminium trichloride at 80℃; under 3677.5 Torr; Hydrogenation.und Erhitzen der bei 190-195grad siedenden Anteile des nach dem Behandeln mit wss.Natriumcarbonat-Loesung erhaltenen Reaktonsprodukts mit wss.Ammoniak und Kupfer(I)-oxid auf 225grad; | |
| With aluminium trichloride at 80℃; under 3677.5 Torr; und Erhitzen der bei 190-195grad siedenden Anteile des nach dem Behandeln mit wss.Natriumcarbonat-Loesung erhaltenen Reaktionsprodukts mit wss.Ammoniak und Kupfer(I)-oxid auf 225grad; |


| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 100% |
| With triethylamine In dichloromethane at 0℃; Reflux; | 58% |
| With triethylamine In chloroform at 0℃; for 1h; | |
| With triethylamine at 0 - 28℃; for 3h; |
-
-
578-54-1
ortho-ethylaniline

-
-
35774-47-1
1-azido-2-ethylbenzene

| Conditions | Yield |
|---|---|
| With tert.-butylnitrite; trimethylsilylazide In acetonitrile at 0 - 20℃; for 1h; | 100% |
| With tert.-butylnitrite; trimethylsilylazide In acetonitrile at 0 - 20℃; for 1h; | 75% |
| With hydrogenchloride; sodium azide; sodium nitrite In water | |
| Stage #1: ortho-ethylaniline With hydrogenchloride; sodium nitrite In water at 0℃; for 0.5h; Stage #2: With sodium azide In water at 0 - 20℃; for 2h; |

| Conditions | Yield |
|---|---|
| With azaphosphatrane salt on Merrifield resin In acetonitrile at 20℃; for 15h; Strecker coupling; | 99% |


| Conditions | Yield |
|---|---|
| With sodium t-butanolate at 110℃; for 18h; | 99% |
| With tetrabutylammomium bromide; palladium diacetate; potassium carbonate; 2,6-bis(diphenylphosphino)pyridine In N,N-dimethyl acetamide at 135℃; for 6h; Catalytic behavior; Buchwald-Hartwig Coupling; Inert atmosphere; | 80% |
| With C22H26Cl2NPPd; sodium t-butanolate In toluene at 110℃; for 22h; Time; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; iodine at 120℃; for 24h; Inert atmosphere; Sealed tube; | 99% |

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; iodine at 120℃; for 24h; Inert atmosphere; Sealed tube; | 99% |
| With 5-ethyl-1,3,7,8-tetramethylalloxazinium triflate; iodine; oxygen In acetonitrile at 60℃; for 24h; Schlenk technique; Green chemistry; | 88% |
-
-
578-54-1
ortho-ethylaniline

-
-
598-21-0
2-Bromoacetyl bromide

-
-
895854-04-3
2-bromo-N-(2'-ethylphenyl)acetamide

| Conditions | Yield |
|---|---|
| With sodium carbonate In water pH=9 - 10; | 99% |
| With triethylamine at 0℃; for 1h; Inert atmosphere; | 86% |
| With sodium carbonate In water for 0.5h; pH=9 - 10; |
-
-
578-54-1
ortho-ethylaniline

| Conditions | Yield |
|---|---|
| Stage #1: ortho-ethylaniline With calcium nitrite In water at 32℃; Acidic conditions; Flow reactor; Stage #2: With lithium bisulfite In water at 70 - 90℃; Stage #3: With hydrogenchloride In water at 120℃; | 98.4% |
-
-
578-54-1
ortho-ethylaniline

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In dichloromethane at 0 - 5℃; Schlenk technique; Inert atmosphere; | 98% |
| With benzyltrimethylammonium tribromide; calcium carbonate In methanol; dichloromethane for 0.5h; Ambient temperature; | 92% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 18h; | 98% |
| With triethylamine In dichloromethane at 0 - 25℃; | 88% |
| With potassium hydrogencarbonate In water | 87% |
-
-
696-59-3
cis,trans-2,5-dimethoxytetrahydrofuran

-
-
578-54-1
ortho-ethylaniline

-
-
299426-84-9
1-(2-ethylphenyl)-1H-pyrrole

| Conditions | Yield |
|---|---|
| With nanosulfated titania In neat (no solvent) at 120℃; for 0.416667h; | 98% |
| With acetic acid | 73% |
| With acetic acid Clauson-Kaas Synthesis; Reflux; |

| Conditions | Yield |
|---|---|
| With sodium t-butanolate; [(η3-cinnamyl)PdCl((t-Bu)2PN=P(i-BuNCH2CH2)3N)] In toluene at 80℃; for 16h; Buchwald-Hartwig amination; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: ortho-ethylaniline With hydrogenchloride; sodium nitrite In water at 0 - 5℃; for 0.5h; Stage #2: acetylacetone With sodium acetate In ethanol; water at 0 - 20℃; for 8h; | 98% |
2-Ethylaniline Consensus Reports
2-Ethylaniline Standards and Recommendations
2-Ethylaniline Specification
The Benzenamine, 2-ethyl-, with the CAS registry number 578-54-1, is also known as 2-Ethylbenzenamine. It belongs to the product categories of Amines; C8; Nitrogen Compounds. Its EINECS number is 209-424-2. This chemical's molecular formula is C8H11N and molecular weight is 121.18. What's more, its systematic name is 2-ethylaniline. It is an important pesticides, dyes and pharmaceutical intermediates, which is also a key synthetic intermediate of new pesticides. Its storage temperature is -20°C.
Physical properties of Benzenamine, 2-ethyl- are: (1)ACD/LogP: 1.93; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.9; (4)ACD/LogD (pH 7.4): 1.93; (5)ACD/BCF (pH 5.5): 16.01; (6)ACD/BCF (pH 7.4): 17.16; (7)ACD/KOC (pH 5.5): 248.38; (8)ACD/KOC (pH 7.4): 266.23; (9)#H bond acceptors: 1; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 26.02 Å2; (13)Index of Refraction: 1.556; (14)Molar Refractivity: 40.03 cm3; (15)Molar Volume: 124.5 cm3; (16)Polarizability: 15.87×10-24cm3; (17)Surface Tension: 38.2 dyne/cm; (18)Density: 0.973 g/cm3; (19)Flash Point: 87.9 °C; (20)Enthalpy of Vaporization: 44.86 kJ/mol; (21)Boiling Point: 212.3 °C at 760 mmHg; (22)Vapour Pressure: 0.174 mmHg at 25°C.
Preparation: this chemical can be prepared by 1-ethyl-2-nitro-benzene at the temperature of 40 °C. This reaction will need reagent H2 and solvent ethanol. This reaction will also need catalyst 1% Pd in AV-17-8-Pd. The yield is about 98%.

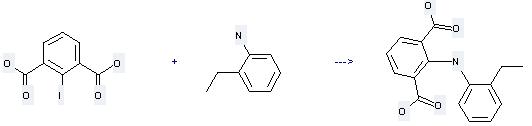
Uses of Benzenamine, 2-ethyl-: it can be used to produce 2-(2-ethyl-phenylamino)-isophthalic acid by heating. It will need reagent N-ethylmorpholine and solvents benzene, various solvent(s) with the reaction time of 1 hour. This reaction will also need catalyst CuCl. The yield is about 65%.
When you are using this chemical, please be cautious about it as the following:
This chemical is toxic by inhalation, in contact with skin and if swallowed. It is irritating to eyes, respiratory system and skin. It has the danger of cumulative effects. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell, you must seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: CCc1ccccc1N
(2)Std. InChI: InChI=1S/C8H11N/c1-2-7-5-3-4-6-8(7)9/h3-6H,2,9H2,1H3
(3)Std. InChIKey: MLPVBIWIRCKMJV-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| bird - wild | LD50 | oral | 750mg/kg (750mg/kg) | Archives of Environmental Contamination and Toxicology. Vol. 12, Pg. 355, 1983. | |
| rat | LD50 | oral | 1260mg/kg (1260mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: CYANOSIS BLOOD: CHANGES IN SPLEEN | Toxicology and Applied Pharmacology. Vol. 22, Pg. 153, 1972. |
Related Products
- 2-Ethylaniline
- 5785-44-4
- 57855-77-3
- 57856-10-7
- 57856-81-2
- 5785-70-6
- 578-57-4
- 5785-81-9
- 578-58-5
- 5786-21-0
- 57864-19-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View