-
Name
2-Fluorotoluene
- EINECS 202-428-5
- CAS No. 95-52-3
- Article Data90
- CAS DataBase
- Density 1.001 g/cm3
- Solubility immiscible with water
- Melting Point -62 °C
- Formula C7H7F
- Boiling Point 116.3 °C at 760 mmHg
- Molecular Weight 110.131
- Flash Point 11.4 °C
- Transport Information UN 2388 3/PG 2
- Appearance clear colorless to slightly yellow liquid
- Safety 16-26-33-36-37/39
- Risk Codes 11-36/37/38
-
Molecular Structure
-
Hazard Symbols
 F,
F, Xi
Xi
- Synonyms Benzene, 1-fluoro-2-methyl-;Toluene, o-fluoro-;1/C7H7F/c1-6-4-2-3-5-7(6)8/h2-5H,1H;1-Methyl-2-fluorobenzene;4-05-00-00799 (Beilstein Handbook Reference);1-fluoro-2-methylbenzene;1-fluoro-2-methyl-benzene;o-Fluorotoluene [UN2388] [Flammable liquid];
- PSA 0.00000
- LogP 2.13410
Synthetic route

| Conditions | Yield |
|---|---|
| With pyridine hydrogenfluoride; sodium nitrite 1.) O deg C, 20 min, 2.) 55 deg C, 1 h; | 99% |
| With hydrogen fluoride; sodium nitrite at -1 - 3℃; for 1h; | 99.5% |
| Stage #1: o-toluidine With hydrogen fluoride at -15 - 0.5℃; Flow reactor; Large scale; Stage #2: With nitrosylsulfuric acid at -5 - 0.5℃; Flow reactor; Large scale; | 98.1% |

| Conditions | Yield |
|---|---|
| With copper(I) trifluoromethanesulfonate bis(trimethylacetonitrile) complex; silver fluoride In N,N-dimethyl-formamide at 140℃; for 22h; Inert atmosphere; | 96% |
-
-
446-48-0
o-fluorobenzyl bromide

-
A
-
95-52-3
2-Fluorotoluene

-
B
-
776-35-2
9,10-dihydrophenanthrene

-
C
-
349-38-2
1,2-bis(2-fluorophenyl)ethane

| Conditions | Yield |
|---|---|
| With magnesium at 600℃; | A 11% B 3% C 80% |

-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
2093-46-1
2-methylphenyl diazonium tetrafluoroborate

-
A
-
95-52-3
2-Fluorotoluene

-
B
-
66107-34-4
2-methylphenyl triflate

| Conditions | Yield |
|---|---|
| at 90℃; for 1h; | A n/a B 75% |

-
-
345-35-7
2-fluorobenzyl chloride

-
A
-
95-52-3
2-Fluorotoluene

-
B
-
776-35-2
9,10-dihydrophenanthrene

-
C
-
349-38-2
1,2-bis(2-fluorophenyl)ethane

| Conditions | Yield |
|---|---|
| With magnesium at 600℃; | A 7% B 5% C 73% |

-
-
19296-22-1
toluene-2-diazonium ; hexafluoro phosphate

-
-
95-52-3
2-Fluorotoluene

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate at 120 - 125℃; for 1h; | 72% |

| Conditions | Yield |
|---|---|
| With 1,3-bis(2,6-diisopropylphenyl)-2,2-difluoro-2,3-dihydro-1h-imidazole; cesium fluoride; diphenylzinc In 1,4-dioxane at 110℃; for 20h; Product distribution / selectivity; Sealed vial; | 72% |
| With 1,3-bis(2,6-diisopropylphenyl)-2,2-difluoro-2,3-dihydro-1h-imidazole; diphenylzinc; cesium fluoride In 1,4-dioxane at 23 - 110℃; for 20.5h; Inert atmosphere; | 87 %Spectr. |
-
-
106-43-4
para-chlorotoluene

-
A
-
352-32-9
p-fluorotoluene

-
B
-
95-52-3
2-Fluorotoluene

-
C
-
352-70-5
m-Fluorotoluene

| Conditions | Yield |
|---|---|
| With Al2CuF8 Gas phase; Inert atmosphere; | A 71% B 5% C 10% |
| With silver fluoride at 350℃; Product distribution / selectivity; Gas phase; | A 20.8% B 0.6% C 35.8% |


| Conditions | Yield |
|---|---|
| With lithium hydroxide monohydrate; silver trifluoromethanesulfonate; Selectfluor In ethyl acetate at 55℃; Sealed tube; | A 71% B n/a |

| Conditions | Yield |
|---|---|
| With Al2CuF8 Gas phase; Inert atmosphere; | A 60% B 7% |
-
-
68971-87-9
(2-tolyl)tributyltin

-
-
95-52-3
2-Fluorotoluene

| Conditions | Yield |
|---|---|
| With copper(II) bis(trifluoromethanesulfonate); tetrabutylammonium triphenyldifluorosilicate In acetonitrile at 60℃; for 3h; Sealed tube; regiospecific reaction; | 50% |
| With copper(I) trifluoromethanesulfonate bis(trimethylacetonitrile) complex; 1-fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate In ethyl acetate at 25℃; for 12h; Inert atmosphere; Glovebox; | 76 %Spectr. |
-
-
546-67-8
lead(IV) tetraacetate

-
-
109-63-7
trifluoroborane diethyl ether

-
-
108-88-3
toluene

-
A
-
352-32-9
p-fluorotoluene

-
B
-
95-52-3
2-Fluorotoluene

-
C
-
352-70-5
m-Fluorotoluene

| Conditions | Yield |
|---|---|
| mercury(II) diacetate Stirring of toluene in BF3*Et2O contg. Pb(OAc)4 and overnight at room temp.; GC anal. 13% recovery of toluene.; | A 43% B 12% C 4% |

-
-
66107-34-4
2-methylphenyl triflate

-
-
95-52-3
2-Fluorotoluene

| Conditions | Yield |
|---|---|
| With bis[(trimethylsilyl)methyl](1,5-cyclooctadiene)palladium(II); t-BuBrettPhos; cesium fluoride In toluene at 130℃; for 12h; | 42% |
| With bis[(trimethylsilyl)methyl](1,5-cyclooctadiene)palladium(II); t-BuBrettPhos; cesium fluoride In toluene at 130℃; for 12h; | 42 %Chromat. |

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In 1,2-dimethoxyethane for 15h; Heating; | A 29% B 45 % Chromat. |
-
-
108-41-8
1-chloro-3-methylbenzene

-
A
-
352-32-9
p-fluorotoluene

-
B
-
95-52-3
2-Fluorotoluene

-
C
-
352-70-5
m-Fluorotoluene

| Conditions | Yield |
|---|---|
| With silver fluoride at 350℃; Product distribution / selectivity; Gas phase; | A 15% B 6.7% C 25.7% |

-
-
95-49-8
2-methylchlorobenzene

-
A
-
352-32-9
p-fluorotoluene

-
B
-
95-52-3
2-Fluorotoluene

-
C
-
352-70-5
m-Fluorotoluene

| Conditions | Yield |
|---|---|
| With silver fluoride at 350℃; Product distribution / selectivity; Gas phase; | A 0.5% B 13.9% C 14.4% |

-
-
462-06-6
fluorobenzene

-
-
593-53-3
Methyl fluoride

-
A
-
352-32-9
p-fluorotoluene

-
B
-
95-52-3
2-Fluorotoluene

-
C
-
352-70-5
m-Fluorotoluene

| Conditions | Yield |
|---|---|
| at 37.5℃; Product distribution; Irradiation; various fluorobenzenes, other conditions; |

-
-
75-45-6
Chlorodifluoromethane

-
A
-
462-06-6
fluorobenzene

-
B
-
352-32-9
p-fluorotoluene

-
C
-
95-52-3
2-Fluorotoluene

-
D
-
352-70-5
m-Fluorotoluene

-
E
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| With methylcyclopentadiene at 650.9℃; Product distribution; Mechanism; conditions of pulse gas compression, other temperatures; |

-
-
107-31-3
Methyl formate

-
-
138850-94-9
C7H7F*H(1+)

-
A
-
95-52-3
2-Fluorotoluene

-
B
-
39014-35-2, 380649-05-8, 52067-01-3
C2H4O2*H(1+)

| Conditions | Yield |
|---|---|
| Thermodynamic data; |


| Conditions | Yield |
|---|---|
| With fluorine In trichlorofluoromethane at -78℃; |

| Conditions | Yield |
|---|---|
| Thermodynamic data; |


-
-
108-88-3
toluene

-
A
-
95-52-3
2-Fluorotoluene

-
B
-
77406-15-6
Toluene; compound with GENERIC INORGANIC NEUTRAL COMPONENT

| Conditions | Yield |
|---|---|
| Thermodynamic data; |


| Conditions | Yield |
|---|---|
| With Selectfluor In acetonitrile for 16h; Heating; also fluorination of other aromatic compounds; | A 20 % Spectr. B 60 % Spectr. |
| With potassium hydroxide; nitrogen; fluorine In methanol at -40℃; Product distribution; different educt and reagent concentration, different temperatures, different fluorine/nitrogen ratio, different solvents, without or with different additives; | A 57.0 % Chromat. B 32.7 % Chromat. |
| With acetyl hypofluorite for 2h; | |
| With trifluorormethanesulfonic acid; Selectfluor In dichloromethane at 40℃; for 24h; Fluorination; Title compound not separated from byproducts; |

| Conditions | Yield |
|---|---|
| With fluorine In trichlorofluoromethane at -78℃; for 1h; Rate constant; Product distribution; competitive reaction with benzene, other solvent; | A 29 % Chromat. B 60 % Chromat. C 11 % Chromat. |
| With lead(IV) acetate; boron trifluoride diethyl etherate; mercury(II) diacetate Ambient temperature; | A 43 % Chromat. B 12 % Chromat. C 4 % Chromat. |
| With caesium fluoroxysulphate; trifluorormethanesulfonic acid In acetonitrile Ambient temperature; Yield given; |

-
-
108-88-3
toluene

-
A
-
352-32-9
p-fluorotoluene

-
B
-
95-52-3
2-Fluorotoluene

-
C
-
352-70-5
m-Fluorotoluene

-
D
-
350-50-5
benzyl fluoride

| Conditions | Yield |
|---|---|
| With acetyl hypofluorite In acetic acid for 0.0833333h; Ambient temperature; Yield given. Yields of byproduct given; | |
| With fluorine In acetonitrile at 0℃; Flow reactor; |

-
-
108-88-3
toluene

-
A
-
352-32-9
p-fluorotoluene

-
B
-
95-52-3
2-Fluorotoluene

-
C
-
352-70-5
m-Fluorotoluene

-
D
-
452-76-6
2,4-difluorotoluene

| Conditions | Yield |
|---|---|
| With tetrafluoroammonium tetrafluoroborate In hydrogen fluoride at 0℃; Further byproducts given. Title compound not separated from byproducts; | A 15 % Spectr. B 15 % Spectr. C 8 % Spectr. D 30 % Spectr. |

-
-
108-88-3
toluene

-
A
-
352-32-9
p-fluorotoluene

-
B
-
95-52-3
2-Fluorotoluene

-
C
-
352-70-5
m-Fluorotoluene

-
D
-
452-76-6
2,4-difluorotoluene

| Conditions | Yield |
|---|---|
| With tetrafluoroammonium tetrafluoroborate In hydrogen fluoride at 0℃; Product distribution; | A 15 % Spectr. B 15 % Spectr. C 8 % Spectr. D 30 % Spectr. E 7 % Spectr. |


| Conditions | Yield |
|---|---|
| With tetrafluoroammonium tetrafluoroborate In hydrogen fluoride at 0℃; Further byproducts given. Title compound not separated from byproducts; | A 15 % Spectr. B 15 % Spectr. C 8 % Spectr. D 7 % Spectr. |


| Conditions | Yield |
|---|---|
| With caesium fluoroxysulphate In acetonitrile at 25℃; Product distribution; Rate constant; reagents ratio, presence of 1,3,5-trinitrobenzene; | A 2 % Chromat. B 4 % Chromat. C 67 % Chromat. |
| With caesium fluoroxysulphate In acetonitrile Ambient temperature; Yield given. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| Stage #1: 2-Fluorotoluene With sec.-butyllithium In tetrahydrofuran at -78℃; for 0.5h; Stage #2: carbon dioxide In tetrahydrofuran under 760.051 Torr; | 100% |
| Stage #1: 2-Fluorotoluene With n-butyllithium Metallation; Stage #2: carbon dioxide Carboxylation; |

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl acetamide at 210℃; for 12h; Microwave irradiation; | 100% |

| Conditions | Yield |
|---|---|
| With triethanolamine; 2,2'-azobis(isobutyronitrile); chlorine at 90 - 115℃; for 5h; | 99% |

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; C11H23N2(1+)*Br4Fe(1-) at 110℃; for 24h; | 98% |

| Conditions | Yield |
|---|---|
| With 1-[2-(diphenylphosphino)phenyl]ethanol; bis(acetylacetonate)nickel(II) In diethyl ether at 20℃; for 5h; | 96% |
| With 1,3-bis(2,6-diisopropylphenyl)-4,5-dihydro-1H-imidazolium tetrafluoroborate; Ni(acac) In tetrahydrofuran at 20℃; for 18h; Kumada-Corriu cross-coupling; | 53 % Chromat. |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Fluorotoluene; phenylmagnesium bromide; 1-[2-(diphenylphosphino)phenyl]ethanol; bis(acetylacetonate)nickel(II) In diethyl ether for 5h; Stage #2: With methanol In diethyl ether Product distribution / selectivity; | 96% |
| Stage #1: 2-Fluorotoluene; phenylmagnesium bromide; bis(acetylacetonate)nickel(II); ((2-hydroxymethyl)phenyl)diphenylphosphine In diethyl ether for 48h; Stage #2: With methanol In diethyl ether Product distribution / selectivity; | 95% |
| With C28H32ClN2NiP In tetrahydrofuran at 20℃; for 24h; Time; Schlenk technique; Inert atmosphere; | 16 %Chromat. |

| Conditions | Yield |
|---|---|
| With C26H24ClN2NiP*0.1C7H8 In toluene at 25℃; for 24h; Kumada Cross-Coupling; Schlenk technique; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With C23H40Br2N2NiO5P; magnesium In tetrahydrofuran at 60℃; for 12h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With Xylaria polymorpha laccase; 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) diammonium salt In 1,4-dioxane at 20℃; for 1.91667h; pH=4.5; Green chemistry; Enzymatic reaction; | 94% |
| With laccase of Pleurotus ostreatus MTCC-1801; 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt In 1,4-dioxane at 20℃; pH=4.5; Enzymatic reaction; | 90% |
| With tert.-butylhydroperoxide; C29H25Cl2N4Ru(1+)*F6P(1-) In acetonitrile at 60℃; for 2h; Schlenk technique; Inert atmosphere; | 74% |

-
-
102-54-5
ferrocene

-
-
95-52-3
2-Fluorotoluene

-
-
165681-07-2
η-pentadienyl-η-1,2-fluoromethylbenzeneiron(II) hexafluorophosphate

| Conditions | Yield |
|---|---|
| With aluminium trichloride; aluminium In water other Radiation; 0.02 mol metal addn. to arene, Fe- and Al-salts mixt. in 1,2,4-C6H3Cl3 (ratio arene:Fe:AlCl3:Al=2:1:2:1.1), homogenization (stirring), mixt. irradiation in microwave oven (850 W) for 3 min, ice addn. (AlCl3 decompn.), filtration, filtrate washing (Et2O); filtration, treating with aq. NH4-salt, ppt. filtration off through sinter, washing (i-PrOH, Et2O); elem. anal.; | 94% |

-
-
95-52-3
2-Fluorotoluene

-
-
824-94-2
p-methoxybenzyl chloride

-
-
53039-52-4
1-methyl-2-(4-methoxybenzyl) benzene

| Conditions | Yield |
|---|---|
| With Ni(PPh3)(1,3-di-tert-butylimidazol-2-ylidene)Br2; magnesium In tetrahydrofuran at 0 - 35℃; for 24h; Schlenk technique; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-methylamino-1-phenylpropan-1-ol With potassium hydroxide In Triethylene glycol dimethyl ether; toluene at 20 - 120℃; for 1h; Large scale; Stage #2: 2-Fluorotoluene In Triethylene glycol dimethyl ether; toluene for 3h; Large scale; Stage #3: (S)-Mandelic acid at 20 - 75℃; for 1h; Temperature; Large scale; | 93% |


| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; 1,3-di-tert-butylimidazolium at 130℃; for 24h; Green chemistry; | 93% |

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; 1,3-di-tert-butylimidazolium at 130℃; for 24h; | 93% |
-
-
95-52-3
2-Fluorotoluene

-
-
63940-51-2
(+/-)-atomoxetine

| Conditions | Yield |
|---|---|
| Stage #1: 3-methylamino-1-phenylpropan-1-ol With potassium hydroxide In dimethyl sulfoxide at 110℃; Stage #2: 2-Fluorotoluene In dimethyl sulfoxide at 145 - 147℃; for 1h; Heating / reflux; | 92.7% |
-
-
95-52-3
2-Fluorotoluene

-
-
13139-86-1
4-methoxyphenyl magnesium bromide

-
-
92495-54-0
4'-methoxy-2-methylbiphenyl

| Conditions | Yield |
|---|---|
| Stage #1: 2-Fluorotoluene With Ni(PPh3)(1,3-di-tert-butylimidazol-2-ylidene)Br2 In tetrahydrofuran at 0℃; for 0.0333333h; Inert atmosphere; Schlenk technique; Stage #2: 4-methoxyphenyl magnesium bromide In tetrahydrofuran at 0 - 25℃; for 10h; Inert atmosphere; Schlenk technique; | 92% |
| With palladium diacetate; DavePhos In 1,2-dimethoxyethane at 80℃; for 12h; | 73% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Fluorotoluene With C78H70Al2Cl4N6P4Rh2; magnesium; ethylene dibromide In tetrahydrofuran at 20℃; for 22h; Inert atmosphere; Glovebox; Stage #2: carbon dioxide In tetrahydrofuran at 20℃; under 760.051 Torr; for 0.5h; | 92% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In chloroform at -5 - 20℃; for 2h; Solvent; Reagent/catalyst; Temperature; | 91% |
| With aluminium trichloride In dichloromethane at 20℃; for 24h; Friedel-Krafts reaction; | 82% |
| With aluminium trichloride | |
| Stage #1: 2-Fluorotoluene; acetyl chloride With aluminum (III) chloride In dichloromethane at 20℃; for 26h; Reflux; Stage #2: With water; sodium carbonate In dichloromethane | |
| With aluminum (III) chloride In dichloromethane at 20℃; for 26h; Reflux; |

| Conditions | Yield |
|---|---|
| With cesium fluoride; lithium hexamethyldisilazane at 110℃; for 12h; Reagent/catalyst; Temperature; Green chemistry; | 90% |
| With cesium fluoride; lithium hexamethyldisilazane at 110℃; for 12h; Reagent/catalyst; Concentration; Sealed tube; Inert atmosphere; | 90% |
-
-
95-52-3
2-Fluorotoluene

-
-
115290-81-8
(R)-N-methyl-3-phenyl-3-hydroxypropylamine

-
-
105314-52-1, 105314-53-2, 63940-51-2, 83015-26-3
(R)-tomoxetine

| Conditions | Yield |
|---|---|
| Stage #1: (R)-N-methyl-3-phenyl-3-hydroxypropylamine With potassium hydroxide In dimethyl sulfoxide at 130℃; for 1h; Stage #2: 2-Fluorotoluene In dimethyl sulfoxide at 130℃; | 89.51% |
| Stage #1: (R)-N-methyl-3-phenyl-3-hydroxypropylamine With sodium hydride In dimethyl sulfoxide for 0.25h; Stage #2: With Potassium benzoate In dimethyl sulfoxide at 50 - 55℃; for 1h; Stage #3: 2-Fluorotoluene In dimethyl sulfoxide at 50 - 120℃; for 6.25 - 7.25h; | |
| With potassium tert-butylate In dimethyl sulfoxide at 60℃; for 8h; |
-
-
95-52-3
2-Fluorotoluene

-
-
4210-32-6
4-tert-butyl benzonitrile

-
-
521295-49-8
2-(4-(tert-butyl)phenyl)-1H-indole

| Conditions | Yield |
|---|---|
| With cesium fluoride; lithium hexamethyldisilazane at 110℃; for 12h; Sealed tube; Inert atmosphere; | 89% |

| Conditions | Yield |
|---|---|
| With 2C2H3F3O*BF3; potassium nitrate at 20℃; for 5h; | 88% |
| With nitric acid | |
| With nitric acid; Nitrogen dioxide | |
| With nitric acid |

| Conditions | Yield |
|---|---|
| With bis(tricyclohexylphosphine)nickel(II) dichloride In tetrahydrofuran at 50℃; for 24h; Inert atmosphere; | 88% |

-
-
95-52-3
2-Fluorotoluene

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-ethane at 90℃; for 18h; Inert atmosphere; | 88% |
-
-
95-52-3
2-Fluorotoluene

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; 2,2,6,6-tetramethylpiperidinyl-lithium at -40℃; for 18h; Glovebox; Inert atmosphere; | 88% |

| Conditions | Yield |
|---|---|
| With cesium fluoride; lithium hexamethyldisilazane In 1,4-dioxane at 110℃; for 12h; Green chemistry; | 88% |
| With cesium fluoride; lithium hexamethyldisilazane In 1,2-dimethoxyethane at 110℃; for 12h; Solvent; Sealed tube; Inert atmosphere; | 88% |
-
-
95-52-3
2-Fluorotoluene

-
-
73183-34-3
bis(pinacol)diborane

-
-
195062-59-0
2-methylphenylboronic acid pinacol ester

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel (0); copper(l) iodide; cesium fluoride; tricyclohexylphosphine In toluene at 80℃; for 24h; Inert atmosphere; | 87.6% |
| With chlorobis(tricyclohexylphosphine)copper(I); cesium fluoride In toluene at 80℃; for 24h; | 79% |
| With tetramethylammonium fluoride; 2-mercaptopyridine sodium salt In acetonitrile at 30 - 35℃; for 24h; Irradiation; Inert atmosphere; | 57% |

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 90℃; for 16h; | 86% |
2-Fluorotoluene Chemical Properties
Molecule structure of o-Fluorotoluene (CAS NO.95-52-3):
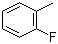
IUPAC Name: 1-Fluoro-2-methylbenzene
Molecular Formula: C7H7F
Molecular Weight: 110.13 g/mol
Density: 1.001 g/cm3
Melting Point: -62 °C
Boiling Point: 116.3 °C at 760 mmHg
Flash Point: 11.4 °C
Index of Refraction: 1.477
Molar Refractivity: 31.07 cm3
Molar Volume: 109.9 cm3
Surface Tension: 27.6 dyne/cm
Enthalpy of Vaporization: 33.99 kJ/mol
Vapour Pressure: 21.8 mmHg at 25 °C
Storage Temp.: flammables area
Water Solubility: immiscible
XLogP3: 2.9
H-Bond Acceptor: 1
Exact Mass: 110.053178
MonoIsotopic Mass: 110.053178
Canonical SMILES: CC1=CC=CC=C1F
InChI: InChI=1S/C7H7F/c1-6-4-2-3-5-7(6)8/h2-5H,1H3
InChIKey: MMZYCBHLNZVROM-UHFFFAOYSA-N
EINECS: 202-428-5
Product Categories: Aromatic Hydrocarbons (substituted) & Derivatives; Organics; Aryl; C7; Halogenated Hydrocarbons
2-Fluorotoluene Uses
o-Fluorotoluene (CAS NO.95-52-3) can be used for intermediates of medicine and pesticide.
2-Fluorotoluene Toxicity Data With Reference
| 1. | orl-brd LD50:100 mg/kg | AECTCV Archives of Environmental Contamination and Toxicology. 12 (1983),355. |
2-Fluorotoluene Consensus Reports
Reported in EPA TSCA Inventory.
2-Fluorotoluene Safety Profile
Hazard Codes:  F,
F,  Xi
Xi
Risk Statements: 11-36/37/38
R11:Highly flammable.
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 16-26-33-36-37/39
S16:Keep away from sources of ignition.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S33:Take precautionary measures against static discharges.
S36:Wear suitable protective clothing.
S37/39:Wear suitable gloves and eye/face protection.
RIDADR: UN 2388 3/PG 2
WGK Germany: 3
RTECS: XT2579000
Hazard Note: Flammable/Irritant
HazardClass: 3
PackingGroup: II
HS Code: 29036990
2-Fluorotoluene Specification
o-Fluorotoluene (CAS NO.95-52-3) is also named as 1-Fluoro-2-methylbenzene ; 1-Methyl-2-fluorobenzene ; 2-Fluorotoluene ; 4-05-00-00799 (Beilstein Handbook Reference) ; BRN 1853362 ; NSC 8859 . o-Fluorotoluene (CAS NO.95-52-3) is clear colorless to slightly yellow liquid with an aromatic odor. It is highly flammable and insoluble in water.
o-Fluorotoluene has generally low reactivity. It reacts with strong oxidizing agents and strong reducing agents. Also it may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. Inhalation of vapor may cause respiratory irritation. Prolonged and repeated vapor exposures may produce systemic toxic effects.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View