-
Name
3,5-Dimethylaniline
- EINECS 203-607-0
- CAS No. 108-69-0
- Article Data71
- CAS DataBase
- Density 0.972 g/cm3
- Solubility <0.1 g/100 mL at 19 °C in water
- Melting Point 7-9 °C
- Formula C8H11N
- Boiling Point 218.5 °C at 760 mmHg
- Molecular Weight 121.182
- Flash Point 93.3 °C
- Transport Information UN 1711 6.1/PG 2
- Appearance clear yellow-brown to brown liquid
- Safety 28-36/37-45-61-28A
- Risk Codes 23/24/25-33-51/53
-
Molecular Structure
-
Hazard Symbols
 T,
T, N
N
- Synonyms 3,5-Dimethylbenzeneamine;3,5-Xylidine;3,5-Dimethy Iani line;3,5-Dimethyl aniline;Product Name: 3,5-dimethylaniline;3,5-Xylidene;3,5-Xylylamine;5-Amino-1,3-xylene;3, 5-Dimethylbenzeneamine;benzenamine, 3,5-dimethyl-;1-Amino-3,5-dimethylbenzene;5-Amino-1,3-dimethylbenzene;Benzene, 1-amino-3,5-dimethyl-;3,5-Xylidine;3, 5-Xylylamine;3,5-Dimethylphenylamine;3,5-Dimethylbenzenamine;
- PSA 26.02000
- LogP 2.46680
Synthetic route

| Conditions | Yield |
|---|---|
| With copper(I) oxide; ammonium hydroxide In 1-methyl-pyrrolidin-2-one at 80℃; for 15h; | 100% |
| With copper(l) iodide; 2-carboxyquinoline N-oxide; potassium carbonate; ammonium hydroxide In dimethyl sulfoxide at 80℃; for 23h; Inert atmosphere; | 91% |
| With copper(l) iodide; ammonia; sodium phosphate In water at 100℃; for 24h; | 86% |
| With diethylenetriaminopentaacetic acid; ammonia; copper(II) oxide; potassium hydroxide In water at 100℃; for 6h; | 74% |
| Stage #1: 5-bromo-1,3-xylene With magnesium In tetrahydrofuran Inert atmosphere; Stage #2: With C10H17NO In tetrahydrofuran; toluene at -78℃; for 2h; Inert atmosphere; Stage #3: With ammonium chloride In tetrahydrofuran; water; toluene Inert atmosphere; | 74% |

| Conditions | Yield |
|---|---|
| With borane-ammonia complex In methanol; water at 49.84℃; for 0.166667h; Catalytic behavior; | 99% |
| With sodium tetrahydroborate In water at 60℃; for 0.0166667h; | 96% |
| Stage #1: 5-nitro-m-xylene With Cu(OH)x impregnated on Fe3O4 In water for 0.0833333h; Green chemistry; Stage #2: With sodium tetrahydroborate In water at 55 - 60℃; for 0.116667h; Green chemistry; | 95% |
-
-
22445-41-6
3,5-dimethylphenyl iodide

-
-
108-69-0
3,5-dimethylaminoaniline

| Conditions | Yield |
|---|---|
| With copper(I) oxide; ammonium hydroxide In 1-methyl-pyrrolidin-2-one at 80℃; for 15h; | 99% |
| With potassium phosphate; copper(l) iodide; N-((1-oxy-pyridin-2-yl)methyl)oxalamic acid; ammonia In water; dimethyl sulfoxide at 25℃; Schlenk technique; Inert atmosphere; Sealed tube; chemoselective reaction; | 95% |
| With copper(l) iodide; 2-carboxyquinoline N-oxide; potassium carbonate; ammonium hydroxide In dimethyl sulfoxide at 50℃; for 23h; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; potassium phosphate In dimethyl sulfoxide at 80℃; UV-irradiation; | 99% |
| Multi-step reaction with 2 steps 1.1: sulfuric acid; nitric acid / 30 °C / Cooling with ice 2.1: palladium on activated charcoal / ethanol / 0.5 h / 50 °C / Autoclave 2.2: 0.83 h / 65 °C / 2250.23 Torr / Autoclave View Scheme |
-
-
62034-72-4
N-hydroxy-3,5-dimethylbenzamide

-
-
108-69-0
3,5-dimethylaminoaniline

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 90℃; for 4h; Lossen Rearrangement; | 97% |

| Conditions | Yield |
|---|---|
| With palladium/alumina; ammonia; hydrogen In toluene at 250℃; under 2250.23 Torr; Temperature; Pressure; Solvent; | 95% |
| With 5%-palladium/activated carbon; hydrazine hydrate; lithium hydroxide In 1,4-dioxane at 170℃; for 16h; Molecular sieve; Inert atmosphere; | 71% |
| With ammonia at 350℃; under 117681 - 183877 Torr; | |
| With aluminum oxide; nitrogen; ammonia; hydrogen at 450℃; under 147102 Torr; |
-
-
101252-04-4
3,5-dimethyl-cyclohexanone oxime

-
-
108-69-0
3,5-dimethylaminoaniline

| Conditions | Yield |
|---|---|
| With Pd(OH)x/LDH In N,N-dimethyl acetamide at 130℃; for 11h; Schlenk technique; Inert atmosphere; | 91% |

-
-
108-69-0
3,5-dimethylaminoaniline

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tris-(trimethylsilyl)silane In toluene at 100℃; Sealed tube; | 88% |
-
-
635-46-1
1,2,3,4-tetrahydroisoquinoline

-
-
99-12-7
5-nitro-m-xylene

-
A
-
91-22-5
quinoline

-
B
-
108-69-0
3,5-dimethylaminoaniline

| Conditions | Yield |
|---|---|
| With nickel-nitrogen-doped carbon framework In water at 145℃; for 18h; Inert atmosphere; Sealed tube; Green chemistry; | A 73% B 76% |

-
-
99-12-7
5-nitro-m-xylene

-
-
6872-06-6
2-methyl indoline

-
A
-
95-20-5
2-methyl-1H-indole

-
B
-
108-69-0
3,5-dimethylaminoaniline

| Conditions | Yield |
|---|---|
| With nickel-nitrogen-doped carbon framework In water at 145℃; for 18h; Inert atmosphere; Sealed tube; Green chemistry; | A 74% B 76% |


| Conditions | Yield |
|---|---|
| With ethene; 5%-palladium/activated carbon; ammonium acetate In acetonitrile at 90℃; under 760.051 Torr; for 15h; Schlenk technique; | 71% |
-
-
64-17-5
ethanol

-
-
99-12-7
5-nitro-m-xylene

-
A
-
102871-67-0
2,5,7-trimethylquinoline

-
B
-
108-69-0
3,5-dimethylaminoaniline

| Conditions | Yield |
|---|---|
| With platinum-loaded TiO2 at 30℃; for 5h; UV-irradiation; | A 68% B 16% |


| Conditions | Yield |
|---|---|
| Stage #1: 2,4-Xylenol With Nonafluorobutanesulfonyl fluoride; tris(trimethylsilyl)amine; potassium hexamethylsilazane In tetrahydrofuran at 60℃; for 5h; Schlenk technique; Inert atmosphere; Stage #2: With hydrogenchloride In water at 20℃; for 0.0833333h; pH=3 - 4; | 67% |
-
-
21857-45-4
5-methoxyindoline

-
-
99-12-7
5-nitro-m-xylene

-
A
-
1006-94-6
5-methoxylindole

-
B
-
108-69-0
3,5-dimethylaminoaniline

| Conditions | Yield |
|---|---|
| With nickel-nitrogen-doped carbon framework In water at 145℃; for 18h; Inert atmosphere; Sealed tube; Green chemistry; | A 62% B 61% |

-
-
108-38-3
m-xylene

-
A
-
108-69-0
3,5-dimethylaminoaniline

-
B
-
95-68-1
2,4-Xylidine

-
C
-
87-62-7
2,6-dimethylaniline

| Conditions | Yield |
|---|---|
| With tris-(2-chloro-ethyl)-amine; trifluorormethanesulfonic acid; trifluoroacetic acid In chloroform at 25℃; for 1h; | A 2% B 58% C 15% |
| With hydrogenchloride; aluminium trichloride; sodium azide In various solvent(s) at 20℃; | |
| With sodium azide; trifluorormethanesulfonic acid at 20℃; Ultrasonic irradiation; | |
| Stage #1: m-xylene With sulfuric acid; hydroxylamine hydrochloride In acetonitrile at 25℃; for 0.166667h; Stage #2: With hydrogenchloride; titanium(III) chloride In water; acetonitrile at 25℃; Inert atmosphere; Sealed tube; Overall yield = 60 percent; Overall yield = 72 mg; |


| Conditions | Yield |
|---|---|
| With hydrogenchloride; PPA; hydroxylamine |

| Conditions | Yield |
|---|---|
| With alkaline sodium hypobromite |
-
-
10272-10-3
2-amino-4,6-dimethylbenzene-1,3-dicarbonitrile

-
A
-
875241-52-4
2-amino-4,6-dimethyl benzamide

-
B
-
108-69-0
3,5-dimethylaminoaniline

| Conditions | Yield |
|---|---|
| With sulfuric acid |
-
-
67543-92-4
3,5-dimethyl-cyclohex-2-enone oxime

-
-
108-69-0
3,5-dimethylaminoaniline

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 170℃; |
-
-
108-68-9
3,5-Dimethylphenol

-
A
-
5369-25-5
bis-(3,5-dimethylphenyl)-amine

-
B
-
108-69-0
3,5-dimethylaminoaniline

| Conditions | Yield |
|---|---|
| With ammonium chloride at 350℃; under 36775.4 Torr; |

-
-
108-69-0
3,5-dimethylaminoaniline

| Conditions | Yield |
|---|---|
| With hydrogenchloride; tin(ll) chloride | |
| With iron; acetic acid | |
| With hydrogenchloride; tin |

| Conditions | Yield |
|---|---|
| at 350℃; under 117681 Torr; |

-
-
108-68-9
3,5-Dimethylphenol

-
A
-
5369-25-5
bis-(3,5-dimethylphenyl)-amine

-
B
-
108-69-0
3,5-dimethylaminoaniline

| Conditions | Yield |
|---|---|
| at 350℃; under 36775.4 - 44130.5 Torr; |

-
-
42205-08-3
1,2,3-triethylbenzene

-
-
83186-90-7
bis-(3,5-dimethyl-cyclohex-2-enylidene)-hydrazine

-
-
108-69-0
3,5-dimethylaminoaniline

-
-
7647-01-0
hydrogenchloride

-
-
91536-77-5
4-(3,5-dimethyl-phenylazo)-3,5-dimethyl-aniline

-
A
-
108-69-0
3,5-dimethylaminoaniline



-
B
-
108-69-0
3,5-dimethylaminoaniline

| Conditions | Yield |
|---|---|
| In chloroform-d1 not isolated; detected by NMR; |

-
-
108-69-0
3,5-dimethylaminoaniline

| Conditions | Yield |
|---|---|
| With 3,6-dimethyl-1,2,4,5-tetrazine; formic acid In acetonitrile at 20℃; for 1h; Reagent/catalyst; Solvent; | |
| With 3,6-dimethyl-1,2,4,5-tetrazine; formic acid In acetonitrile at 20℃; for 1h; Reagent/catalyst; Solvent; |

| Conditions | Yield |
|---|---|
| With pyridine In acetonitrile at 20℃; for 1h; Reagent/catalyst; |

-
-
108-69-0
3,5-dimethylaminoaniline

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
114097-28-8
N-(3,5-dimethylphenyl)-4-methyl-benzenesulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 4℃; | 100% |
| With silica gel at 20℃; | 95% |
| With triethylamine In dichloromethane at 0 - 20℃; | 82% |
-
-
108-69-0
3,5-dimethylaminoaniline

-
-
75-98-9
Trimethylacetic acid

-
-
86489-67-0
N-(3,5-dimethylphenyl)pivalamide

| Conditions | Yield |
|---|---|
| With diphosphorus tetraiodide In tetrachloromethane; dichloromethane Heating; | 100% |
-
-
108-69-0
3,5-dimethylaminoaniline

-
-
762-42-5
dimethyl acetylenedicarboxylate

-
-
92952-51-7
dimethyl (Z)-N-(3,5-dimethylphenyl)aminofumarate

| Conditions | Yield |
|---|---|
| at 20℃; for 0.133333h; Michael addition; | 100% |
| In diethyl ether at 0℃; | 75% |
-
-
123-11-5
4-methoxy-benzaldehyde

-
-
108-69-0
3,5-dimethylaminoaniline

-
-
199435-31-9
3,5-dimethyl-N-(4-methoxybenzylidene)aniline

| Conditions | Yield |
|---|---|
| at 20℃; | 100% |
| With acetic acid In methanol at 20℃; |
-
-
4755-77-5
Ethyl oxalyl chloride

-
-
108-69-0
3,5-dimethylaminoaniline

-
-
333441-77-3
2-(3,5-dimethylphenylamino)-2-oxoacetic acidethyl ester

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 1.5h; Inert atmosphere; | 100% |
| With triethylamine In tetrahydrofuran at 0 - 20℃; | 68% |
-
-
108-69-0
3,5-dimethylaminoaniline

-
-
857070-85-0
3-(3,5-dimethyl-phenylamino)-propionamide

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 80℃; | 100% |
-
-
864460-87-7
2-(2-tert-butoxycarbonylaminopyridin-4-ylmethylthio)pyridine-3-carboxylic acid

-
-
108-69-0
3,5-dimethylaminoaniline

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 20℃; for 12h; | 100% |
-
-
1009646-49-4
{4-[6-formyl-1-(3-hydroxy-6-methylpyridin-2-ylmethyl)-1H-benzoimidazol-2-ylamino]piperidin-1-yl}acetic acid ethyl ester

-
-
108-69-0
3,5-dimethylaminoaniline

-
-
1009646-54-1
{4-[6-[(3,5-dimethylphenylamino)methyl]-1-(3-hydroxy-6-methylpyridin-2-ylmethyl)-1H-benzoimidazol-2-ylamino]piperidin-1-yl}acetic acid ethyl ester

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride; acetic acid In acetonitrile at 20℃; for 12h; | 100% |
-
-
108-69-0
3,5-dimethylaminoaniline

-
-
583-53-9
1,2-dibromobenzene

-
-
1068437-89-7
N,N'-di(3,5-dimethylphenyl)benzene-1,2-diamine

| Conditions | Yield |
|---|---|
| With ammonium chloride; sodium t-butanolate; palladium diacetate In water; argon; toluene | 100% |
| With tri-tert-butyl phosphine; palladium diacetate; sodium t-butanolate In toluene at 110℃; for 14h; Inert atmosphere; | 100 %Chromat. |
-
-
37630-26-5, 4735-49-3
β-(1-naphthyl)nitroethylene

-
-
108-69-0
3,5-dimethylaminoaniline

-
-
1268482-47-8
C20H20N2O2

| Conditions | Yield |
|---|---|
| at 20℃; for 0.1h; Michael addition; neat (no solvent); | 100% |
-
-
5670-66-6, 5670-67-7, 3156-39-6
1-nitro-2-(2-nitrovinyl)benzene

-
-
108-69-0
3,5-dimethylaminoaniline

-
-
1268482-40-1
C16H17N3O4

| Conditions | Yield |
|---|---|
| at 20℃; for 0.1h; Michael addition; neat (no solvent); | 100% |

| Conditions | Yield |
|---|---|
| at 20℃; for 0.1h; Michael addition; neat (no solvent); | 100% |
-
-
882-26-8
3-nitro-β-nitrostyrene

-
-
108-69-0
3,5-dimethylaminoaniline

-
-
108836-94-8
3,5-dimethyl-N-[2-nitro-1-(3-nitro-phenyl)-ethyl]-aniline

| Conditions | Yield |
|---|---|
| at 20℃; for 0.1h; Michael addition; neat (no solvent); | 100% |
-
-
706-07-0
1-chloro-4-(2-nitrovinyl)benzene

-
-
108-69-0
3,5-dimethylaminoaniline

-
-
1268482-45-6
C16H17ClN2O2

| Conditions | Yield |
|---|---|
| at 20℃; for 0.1h; Michael addition; neat (no solvent); | 100% |
-
-
108-69-0
3,5-dimethylaminoaniline

-
-
762-21-0
acetylenedicarboxylic acid diethyl ester

-
-
1349802-62-5
diethyl (Z)-N-(3,5-dimethylphenyl)aminofumarate

| Conditions | Yield |
|---|---|
| at 20℃; for 0.15h; Michael addition; | 100% |
-
-
100-52-7
benzaldehyde

-
-
108-69-0
3,5-dimethylaminoaniline

-
-
91000-43-0
3,5-dimethyl-N-benzylideneaniline

| Conditions | Yield |
|---|---|
| at 20℃; | 100% |
| In methanol | 99% |
| In ethanol for 3h; Reflux; |
-
-
104-87-0
4-methyl-benzaldehyde

-
-
108-69-0
3,5-dimethylaminoaniline

-
-
107399-20-2
3,5-dimethyl-N-(4-methylbenzylidene)aniline

| Conditions | Yield |
|---|---|
| at 20℃; | 100% |
-
-
104-88-1
4-chlorobenzaldehyde

-
-
108-69-0
3,5-dimethylaminoaniline

-
-
180569-73-7
3,5-dimethyl-N-(4-chlorobenzylidene)aniline

| Conditions | Yield |
|---|---|
| at 20℃; | 100% |
| In neat (no solvent) at 20℃; for 0.5h; Schlenk technique; Molecular sieve; Inert atmosphere; |
-
-
108-69-0
3,5-dimethylaminoaniline

-
-
1122-91-4
4-bromo-benzaldehyde

-
-
348601-49-0
3,5-dimethyl-N-(4-bromobenzylidene)aniline

| Conditions | Yield |
|---|---|
| at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| at 20℃; | 100% |
-
-
26074-52-2
2-bromo-butyryl bromide

-
-
108-69-0
3,5-dimethylaminoaniline

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; | 100% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 14h; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 20℃; Inert atmosphere; | 99% |
| Stage #1: acetic anhydride; 3,5-dimethylaminoaniline In tetrahydrofuran at 0 - 20℃; Inert atmosphere; Stage #2: With sodium hydroxide In tetrahydrofuran; water pH=12 - 14; Cooling with ice; | 99% |
| Stage #1: acetic anhydride; 3,5-dimethylaminoaniline In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Stage #2: With sodium hydroxide In tetrahydrofuran; water pH=12 - 14; Cooling with ice; | 99% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 201℃; for 1h; | 99% |
| Stage #1: 3,5-dimethylaminoaniline With potassium carbonate In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: acetyl chloride for 2h; Inert atmosphere; | 99% |
| With potassium carbonate In dichloromethane at 20℃; for 2.5h; Cooling with ice; | 98.8% |
-
-
156-38-7
4-hydroxyphenylacetate

-
-
108-69-0
3,5-dimethylaminoaniline

-
-
131179-77-6
N-(3,5-dimethylphenyl)-4-hydroxyphenylacetamide

| Conditions | Yield |
|---|---|
| With fluorosulfonyl fluoride; N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 5h; | 99% |
| With boric acid In toluene for 18h; Heating; | 95% |
| With pyridine; FI-750-M; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In water at 60℃; for 0.75h; Sealed tube; | 86% |
-
-
2009-81-6
2-dimethylaminomethylene-1,3-bis(dimethylimonio)propane diperchlorate

-
-
108-69-0
3,5-dimethylaminoaniline

| Conditions | Yield |
|---|---|
| With perchloric acid In ethanol at 105℃; for 1.5h; | 99% |
-
-
15761-39-4
1-(tert-butoxycarbonyl)-L-proline

-
-
108-69-0
3,5-dimethylaminoaniline

-
-
137496-70-9
(S)-tert-butyl 2-((3,5-dimethylphenyl)carbamoyl)pyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With chloroformic acid ethyl ester; triethylamine In dichloromethane at 20℃; for 1h; | 99% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 12h; | 85% |
| With benzotriazol-1-ol; dicyclohexyl-carbodiimide In dichloromethane for 12h; Ambient temperature; | 65% |
-
-
106-43-4
para-chlorotoluene

-
-
108-69-0
3,5-dimethylaminoaniline

-
-
34160-16-2
3,5-dimethyl-N-p-tolylbenzenamine

| Conditions | Yield |
|---|---|
| With bis(η3-allyl-μ-chloropalladium(II)); potassium tert-butylate; 1,3-bis[(2,6-diisopropyl)phenyl]imidazolinium chloride In 1,4-dioxane at 100℃; for 1.5h; Inert atmosphere; | 99% |
| With bis[chloro(1,2,3-trihapto-allylbenzene)palladium(II)]; N-[2-(di(1-adamantyl)phosphino)phenyl]morpholine; potassium hydroxide; sodium t-butanolate In water at 110℃; for 22h; Buchwald-Hartwig amination; Inert atmosphere; chemoselective reaction; | 95% |
| With sodium t-butanolate; [Na2PdCl4]; dicyclohexyl-(9-ethyl-9H-fluoren-9-yl)phosphane*HBF4 In toluene at 120℃; for 12h; Buchwald-Hartwig coupling reaction; | |
| With sodium t-butanolate; palladium diacetate; dicyclohexyl(9-ethyl-9H-fluorene-9-yl)-phosphine·HBF4 In toluene at 120℃; for 12h; Product distribution / selectivity; Buchwald-Hartwig Amination; |
3,5-Dimethylaniline Chemical Properties
Molar mass:121.17964g/mol
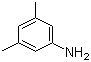
Structure:
Synonyms :3,5-Xylidene;3,5-Dimethylbenzenamine;Benzenamine, 3,5-dimethyl-;5-Amino-1,3-xylene;3,5-Xylylamine;5-Amino-m-xylene;m-Xylidine
Density:0.975 g/cm3
Flash Point:93.3 °C
Boiling Point:218.5 °C at 760 mmHg
Index of Refraction:1.559
Vapour Pressure:0.126 mmHg at 25°C
Melting point:7-9°C
Solubility: Not dissolve in water
Water solubility:<0.1 g/100 mL at 19°C
3,5-Dimethylaniline Uses
3,5-Dimethylaniline Toxicity Data With Reference
| 1. | mmo-sat 1 mg/plate | EMMUEG Environmental and Molecular Mutagenesis. 11 (Suppl 12)(1988),1. | ||
| 2. | orl-rat LD50:707 mg/kg | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) PB214-270 . | ||
| 3. | orl-mus LD50:421 mg/kg | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) PB214-270 . |
Other: See actual entry in RTECS for complete information.
3,5-Dimethylaniline Consensus Reports
3,5-Dimethylaniline Safety Profile
Suspected carcinogen. Moderately toxic by ingestion. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx. See also XYLIDINE; 2,3-XYLIDINE; 2,4-XYLIDINE; 2,5-XYLIDINE; 2,6-XYLIDINE; 3,4-XYLIDINE; 2,4-XYLIDINE HYDROCHLORIDE; 2,5-XYLIDINE HYDROCHLORIDE.
Hazard Codes:T ,N
,N
Risk Statements:
23: Toxic by inhalation
24: Toxic in contact with skin
25: Toxic if swallowed
33: Danger of cumulative effects
51: Toxic to aquatic organisms
53: May cause long-term adverse effects in the aquatic environment
28: After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer)
37: Wear suitable gloves
39: Wear eye/face protection
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
61: Avoid release to the environment. Refer to special instructions safety data sheet
3,5-Dimethylaniline Standards and Recommendations
3,5-Dimethylaniline Specification
Conditions to Avoid:Incompatible materials, light, exposure to air, excess heat.
Chemical Stability:Stable under normal pressures and temperatures. Darkens on exposure to light and air.
Incompatibilities with Other Materials: Excess heat, chloroformates, acids, acid chlorides,acid anhydrides,halogens,coatings, plastics, rubber, hypochlorite.
Hazardous Decomposition Products: carbon dioxide,nitrogen oxides,Carbon monoxide.
Related Products
- 3,10-Diaminotricyclo(5.2.1.0(sup 2,6))decane
- 3,10-Dinitrophenanthrene
- 3-((10-ETHYL-11-(p-HYDROXYPHENYL)DIBENZ-(B,F)OXEPIN-3-YL)OXY)-1,2-PROPANEDIOL HYDRATE (4:1)
- 3-(1,1,2,2-Tetrafluoroethoxy)aniline
- 3-(1,1,2,2-Tetrafluoroethoxy)benzaldehyde
- 3-(1,1,2,2-Tetrafluoroethoxy)bromobenzene
- 3-(1,1,2,2-Tetrafluoroethoxy)toluene
- 3-[1,1'-Biphenyl]-4-yl-1,2,3,4-tetrahydro-1-naphthol
- 3,11-Dichloro-6,11-dihydro-6-methyldibenzo[c,f][1,2]thiazepine 5,5-dioxide
- 3-[(1,1-Dimethyl-2-hydroxyethyl)amino]-2-hydroxypropanesulfonicacid
- 108694-93-5
- 108695-16-5
- 108695-25-6
- 108698-02-8
- 1087-02-1
- 108-70-3
- 108710-70-9
- 108-71-4
- 108714-73-4
- 108723-79-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View