-
Name
3-Fluorotoluene
- EINECS 206-524-8
- CAS No. 352-70-5
- Article Data76
- CAS DataBase
- Density 1.001 g/cm3
- Solubility immiscible with water
- Melting Point -87 °C
- Formula C7H7F
- Boiling Point 118.2 °C at 760 mmHg
- Molecular Weight 110.131
- Flash Point 9.4 °C
- Transport Information UN 2388 3/PG 2
- Appearance clear colorless to light yellow liquid
- Safety 16-26-36-37/39
- Risk Codes 11-36/37/38
-
Molecular Structure
-
Hazard Symbols
 F,
F,  Xi
Xi
- Synonyms Toluene, m-fluoro- (8CI);m-Fluorotoluene;Toluene, m-fluoro-;1-chloro-4-fluoro-benzene;1-Fluoro-3-methylbenzene;1-Methyl-3-fluorobenzene;Benzene, 1-fluoro-3-methyl-;m-Fluorotoluene [UN2388] [Flammable liquid];
- PSA 0.00000
- LogP 2.13410
Synthetic route

| Conditions | Yield |
|---|---|
| With pyridine hydrogenfluoride; sodium nitrite 1.) O deg C, 20 min, 2.) 70 deg C, 1 h; | 98% |
| Stage #1: 1-amino-3-methylbenzene With hydrogen fluoride at -5 - 0.5℃; Flow reactor; Large scale; Stage #2: With nitrosylsulfuric acid at 0.5 - 2℃; Flow reactor; Large scale; | 98.3% |
| Stage #1: 1-amino-3-methylbenzene With hydrogen fluoride at 0℃; for 3h; Stage #2: With sodium nitrite at -5 - 35℃; for 4h; | 90.1% |
-
-
1202873-16-2
3-fluoro-1-methyl-2,5-cyclohexadiene-1-carboxylic acid

-
-
352-70-5
m-Fluorotoluene

| Conditions | Yield |
|---|---|
| With chlorosulfonic acid In dichloromethane at 0℃; for 0.166667h; | 82% |
-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
1422-76-0
3-methylbenzenediazonium tetrafluoroborate

-
A
-
352-70-5
m-Fluorotoluene

-
B
-
32578-31-7
3-tolyl triflate

| Conditions | Yield |
|---|---|
| at 90℃; for 1h; | A n/a B 75% |

-
-
106-43-4
para-chlorotoluene

-
A
-
352-32-9
p-fluorotoluene

-
B
-
95-52-3
2-Fluorotoluene

-
C
-
352-70-5
m-Fluorotoluene

| Conditions | Yield |
|---|---|
| With Al2CuF8 Gas phase; Inert atmosphere; | A 71% B 5% C 10% |
| With silver fluoride at 350℃; Product distribution / selectivity; Gas phase; | A 20.8% B 0.6% C 35.8% |

-
-
456-41-7
1-(Bromomethyl)-3-fluorobenzene

-
A
-
352-70-5
m-Fluorotoluene

-
B
-
351-22-4
1,2-bis(3-fluorophenyl)ethane

| Conditions | Yield |
|---|---|
| With magnesium at 600℃; | A 12% B 67% |

-
-
352-70-5
m-Fluorotoluene

| Conditions | Yield |
|---|---|
| With lithium hydroxide monohydrate; silver trifluoromethanesulfonate; Selectfluor In ethyl acetate at 55℃; Sealed tube; | 66% |
| With copper(I) trifluoromethanesulfonate bis(trimethylacetonitrile) complex; 1-fluoro-2,4,6-trimethylpyridinium trifluoromethanesulfonate In ethyl acetate at 40℃; for 12h; Inert atmosphere; Glovebox; | 67 %Spectr. |

-
-
352-70-5
m-Fluorotoluene

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate at 120 - 125℃; for 1h; | 65% |

| Conditions | Yield |
|---|---|
| With Al2CuF8 Gas phase; Inert atmosphere; | 61% |

| Conditions | Yield |
|---|---|
| With Al2CuF8 Gas phase; Inert atmosphere; | A 60% B 7% |

| Conditions | Yield |
|---|---|
| Stage #1: m-Toluic acid With tetrakis(acetonitrile)copper(I)tetrafluoroborate; tetrabutylammonium tetra(tert-butyl alcohol) coordinate fluoride; copper(II) bis(trifluoromethanesulfonate) In acetonitrile at 23℃; for 0.5h; Sealed tube; Inert atmosphere; Stage #2: In acetonitrile at 35℃; Irradiation; Sealed tube; Inert atmosphere; | 57% |
-
-
546-67-8
lead(IV) tetraacetate

-
-
109-63-7
trifluoroborane diethyl ether

-
-
108-88-3
toluene

-
A
-
352-32-9
p-fluorotoluene

-
B
-
95-52-3
2-Fluorotoluene

-
C
-
352-70-5
m-Fluorotoluene

| Conditions | Yield |
|---|---|
| mercury(II) diacetate Stirring of toluene in BF3*Et2O contg. Pb(OAc)4 and overnight at room temp.; GC anal. 13% recovery of toluene.; | A 43% B 12% C 4% |

-
-
108-41-8
1-chloro-3-methylbenzene

-
A
-
352-32-9
p-fluorotoluene

-
B
-
95-52-3
2-Fluorotoluene

-
C
-
352-70-5
m-Fluorotoluene

| Conditions | Yield |
|---|---|
| With silver fluoride at 350℃; Product distribution / selectivity; Gas phase; | A 15% B 6.7% C 25.7% |

-
-
920-66-1
1,1,1,3',3',3'-hexafluoro-propanol

-
-
1422-76-0
3-methylbenzenediazonium tetrafluoroborate

-
A
-
352-70-5
m-Fluorotoluene

| Conditions | Yield |
|---|---|
| at 20℃; Irradiation; | A 15% B 85 % Chromat. |

-
-
95-49-8
2-methylchlorobenzene

-
A
-
352-32-9
p-fluorotoluene

-
B
-
95-52-3
2-Fluorotoluene

-
C
-
352-70-5
m-Fluorotoluene

| Conditions | Yield |
|---|---|
| With silver fluoride at 350℃; Product distribution / selectivity; Gas phase; | A 0.5% B 13.9% C 14.4% |

-
-
462-06-6
fluorobenzene

-
-
593-53-3
Methyl fluoride

-
A
-
352-32-9
p-fluorotoluene

-
B
-
95-52-3
2-Fluorotoluene

-
C
-
352-70-5
m-Fluorotoluene

| Conditions | Yield |
|---|---|
| at 37.5℃; Product distribution; Irradiation; various fluorobenzenes, other conditions; |

-
-
75-45-6
Chlorodifluoromethane

-
A
-
462-06-6
fluorobenzene

-
B
-
352-32-9
p-fluorotoluene

-
C
-
95-52-3
2-Fluorotoluene

-
D
-
352-70-5
m-Fluorotoluene

-
E
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| With methylcyclopentadiene at 650.9℃; Product distribution; Mechanism; conditions of pulse gas compression, other temperatures; |

-
-
624-31-7
4-tolyl iodide

-
A
-
352-70-5
m-Fluorotoluene

-
B
-
108-41-8
1-chloro-3-methylbenzene

-
C
-
591-17-3
meta-bromotoluene

-
D
-
106-43-4
para-chlorotoluene

-
E
-
106-38-7
para-bromotoluene

-
F
-
108-39-4
3-methyl-phenol

| Conditions | Yield |
|---|---|
| With potassium hydroxide; potassium chloride; sodium fluoride; potassium bromide In water at 308℃; Mechanism; Product distribution; | A 1.37 % Chromat. B 1.67 % Chromat. C 2.66 % Chromat. D 1.44 % Chromat. E 2.22 % Chromat. F 2.88 % Chromat. |

-
-
624-31-7
4-tolyl iodide

-
A
-
106-44-5
p-cresol

-
B
-
352-32-9
p-fluorotoluene

-
C
-
352-70-5
m-Fluorotoluene

-
D
-
108-41-8
1-chloro-3-methylbenzene

-
E
-
108-39-4
3-methyl-phenol

-
F
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| With potassium hydroxide; potassium chloride; sodium fluoride In water at 312℃; Mechanism; Product distribution; | A 13.0 % Chromat. B 5.01 % Chromat. C 5.97 % Chromat. D 3.0 % Chromat. E 18.6 % Chromat. F 2.6 % Chromat. |

-
-
28490-56-4
1-fluoro-3-(iodomethyl)benzene

-
-
352-70-5
m-Fluorotoluene

| Conditions | Yield |
|---|---|
| With Perbenzoic acid; tri-n-butyl-tin hydride In benzene at 90℃; for 12h; Mechanism; in the presence of α-iodotoluene (competitor), relative reactivity; |

| Conditions | Yield |
|---|---|
| Thermodynamic data; |


-
-
108-88-3
toluene

-
A
-
352-70-5
m-Fluorotoluene

-
B
-
77406-15-6
Toluene; compound with GENERIC INORGANIC NEUTRAL COMPONENT

| Conditions | Yield |
|---|---|
| Thermodynamic data; |


| Conditions | Yield |
|---|---|
| With fluorine In trichlorofluoromethane at -78℃; for 1h; Rate constant; Product distribution; competitive reaction with benzene, other solvent; | A 29 % Chromat. B 60 % Chromat. C 11 % Chromat. |
| With lead(IV) acetate; boron trifluoride diethyl etherate; mercury(II) diacetate Ambient temperature; | A 43 % Chromat. B 12 % Chromat. C 4 % Chromat. |
| With caesium fluoroxysulphate; trifluorormethanesulfonic acid In acetonitrile Ambient temperature; Yield given; |

-
-
108-88-3
toluene

-
A
-
352-32-9
p-fluorotoluene

-
B
-
95-52-3
2-Fluorotoluene

-
C
-
352-70-5
m-Fluorotoluene

-
D
-
350-50-5
benzyl fluoride

| Conditions | Yield |
|---|---|
| With acetyl hypofluorite In acetic acid for 0.0833333h; Ambient temperature; Yield given. Yields of byproduct given; | |
| With fluorine In acetonitrile at 0℃; Flow reactor; |

-
-
108-88-3
toluene

-
A
-
352-32-9
p-fluorotoluene

-
B
-
95-52-3
2-Fluorotoluene

-
C
-
352-70-5
m-Fluorotoluene

-
D
-
452-76-6
2,4-difluorotoluene

| Conditions | Yield |
|---|---|
| With tetrafluoroammonium tetrafluoroborate In hydrogen fluoride at 0℃; Further byproducts given. Title compound not separated from byproducts; | A 15 % Spectr. B 15 % Spectr. C 8 % Spectr. D 30 % Spectr. |

-
-
108-88-3
toluene

-
A
-
352-32-9
p-fluorotoluene

-
B
-
95-52-3
2-Fluorotoluene

-
C
-
352-70-5
m-Fluorotoluene

-
D
-
452-76-6
2,4-difluorotoluene

| Conditions | Yield |
|---|---|
| With tetrafluoroammonium tetrafluoroborate In hydrogen fluoride at 0℃; Product distribution; | A 15 % Spectr. B 15 % Spectr. C 8 % Spectr. D 30 % Spectr. E 7 % Spectr. |


| Conditions | Yield |
|---|---|
| With tetrafluoroammonium tetrafluoroborate In hydrogen fluoride at 0℃; Further byproducts given. Title compound not separated from byproducts; | A 15 % Spectr. B 15 % Spectr. C 8 % Spectr. D 7 % Spectr. |


| Conditions | Yield |
|---|---|
| With Perbenzoic acid; tri-n-butyl-tin hydride In benzene at 90℃; for 12h; Mechanism; in the presence of α-bromotoluene, α-iodotoluene, or α,3-dichlorotoluene (20 h) (competitors), relative reactivities; |

| Conditions | Yield |
|---|---|
| With Perbenzoic acid; tri-n-butyl-tin hydride In benzene at 90℃; for 12h; Mechanism; in the presence of α-chlorotoluene (competitor), relative reactivity; |

| Conditions | Yield |
|---|---|
| With oxygen; pyridinium chlorochromate In water at 120℃; under 45004.5 Torr; for 2h; | 95% |
| With copper(l) iodide; oxygen; nitric acid at 160℃; under 11251.1 - 18751.9 Torr; for 4.7h; Autoclave; | 84.7% |
| With cerium(III) chloride; 1,1,1-trichloroethanol; oxygen In acetonitrile at 60℃; Irradiation; | 83% |
-
-
352-70-5
m-Fluorotoluene

-
-
199620-14-9, 13007-92-6
chromium(0) hexacarbonyl

-
-
33411-10-8
(η6-1-fluoro-3-methylbenzene)tricarbonylchromium(0)

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; dibutyl ether heating, 8d; filtration; evapn.; chromy. (silica, ether/petroleum ether); recrystn. (petroleum ether/ether); elem. anal.; | 95% |
| In tetrahydrofuran; dibutyl ether at 160℃; | 80% |
| In tetrahydrofuran; dibutyl ether Cr(CO)6 and arene were heated under reflux in a mixt. of n-Bu2O and THFfor 42 h under N2 atmosphere; soln. was cooled, filtered, evapd. under reduced pressure, residue was dissolved in ether, refiltered, light petroleum was added to the filtrate, ppt. collected, recrystd. from ether-light petroleum; elem. anal.; | 49% |
| In tetrahydrofuran; dibutyl ether reflux for 48 h; filtration through Celite, evapn. under reduced pressure, recrystn. from ether/petroleum ether; | 42% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl acetamide at 210℃; for 12h; Microwave irradiation; | 92% |

| Conditions | Yield |
|---|---|
| With dichloro bis(acetonitrile) palladium(II); potassium carbonate; Trimethylacetic acid In N,N-dimethyl acetamide at 120℃; for 3h; regioselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| With copper diacetate; 1,8-diazabicyclo[5.4.0]undec-7-ene In 1,4-dioxane at 80℃; for 10h; | 91% |

| Conditions | Yield |
|---|---|
| Stage #1: m-Fluorotoluene With C78H70Al2Cl4N6P4Rh2; magnesium; ethylene dibromide In tetrahydrofuran at -30℃; for 22h; Inert atmosphere; Glovebox; Stage #2: carbon dioxide In tetrahydrofuran at 20℃; under 760.051 Torr; for 0.5h; | 90% |

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; 1,3-di-tert-butylimidazolium at 130℃; for 24h; Green chemistry; | 90% |
-
-
100-02-7
4-nitro-phenol

-
-
352-70-5
m-Fluorotoluene

-
-
630412-56-5
1-fluoro-3-((4-nitrophenoxy)methyl)benzene

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; 1,3-di-tert-butylimidazolium at 130℃; for 24h; | 90% |

| Conditions | Yield |
|---|---|
| With silver(I) hexafluorophosphate; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; C17H21O3P In tetrahydrofuran at 120℃; for 20h; Inert atmosphere; Molecular sieve; | 89% |
| With dichloro(benzene)ruthenium(II) dimer; silver trifluoromethanesulfonate; P(p-C6H4F)3 In 1,4-dioxane for 24h; Inert atmosphere; Molecular sieve; Reflux; | 83% |
| With triethylsilane; [bis(2-methylallyl)cycloocta-1,5-diene]ruthenium(II); trifluorormethanesulfonic acid; 1,5-bis-(diphenylphosphino)pentane; triethylamine In 1,4-dioxane for 24h; Inert atmosphere; Reflux; | 79% |
-
-
23866-67-3
9-(2-pyridyl)carbazole

-
-
352-70-5
m-Fluorotoluene

| Conditions | Yield |
|---|---|
| With N-hydroxyphthalimide; oxygen; palladium diacetate at 80℃; under 760.051 Torr; for 24h; Schlenk technique; regioselective reaction; | 89% |

| Conditions | Yield |
|---|---|
| With aluminium trichloride at 25℃; for 2h; | 88% |
-
-
352-70-5
m-Fluorotoluene

-
-
74483-52-6
4-fluoro-3-methylbenzotrifluoride

| Conditions | Yield |
|---|---|
| 87% |

| Conditions | Yield |
|---|---|
| Stage #1: C25H38O3Si2 With lithium diisopropyl amide In tetrahydrofuran at 0 - 3℃; for 2h; Stage #2: m-Fluorotoluene In tetrahydrofuran at 20℃; for 24h; | 87% |

| Conditions | Yield |
|---|---|
| With Selectfluor; trifluoroacetic acid In acetonitrile at 80℃; for 12h; | 86% |
| With dipotassium peroxodisulfate at 110℃; for 12h; Schlenk technique; | 75% |
| With Selectfluor; trifluoroacetic acid In acetonitrile at 80℃; for 10h; | 61% |

| Conditions | Yield |
|---|---|
| With nitric acid at -15 - 55℃; for 8.5h; Inert atmosphere; | 85% |
| With nitric acid In water at 0 - 5℃; for 0.5h; | 38% |
| With nitric acid |

| Conditions | Yield |
|---|---|
| In methanol at -10 - 20℃; Green chemistry; Industrial scale; | 85% |

-
-
352-70-5
m-Fluorotoluene

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; 2,2,6,6-tetramethylpiperidinyl-lithium at -40℃; for 18h; Glovebox; Inert atmosphere; | 84% |
-
-
352-70-5
m-Fluorotoluene

-
A
-
349-01-9
1-fluoro-5-methyl-2,4-dinitro-benzene

-
B
-
110600-90-3
1-fluoro-3-methyl-2,4-dinitro-benzene

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid 1.) not over 35 deg C; 2.) 30 min, room temp.; | A 81% B n/a |
| With sulfuric acid; nitric acid at 0 - 35℃; for 0.5h; | A 36% B n/a |
| With sulfuric acid; nitric acid at 35℃; for 0.5h; Cooling with ice; Overall yield = 32 g; | A 36% B n/a |
-
-
352-70-5
m-Fluorotoluene

-
-
1109-15-5
tris(pentafluorophenyl)borate

-
-
6016-04-2
N,N’-di(3-pyridyl)-1,4,5,8-naphthalenetetracarboxydiimide

| Conditions | Yield |
|---|---|
| In benzene at 80℃; | 80% |

-
-
352-70-5
m-Fluorotoluene

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; palladium diacetate; iron(II) chloride at 110℃; for 10h; Inert atmosphere; regioselective reaction; | 80% |

| Conditions | Yield |
|---|---|
| With Xantphos-Pd-G3; potassium hexamethylsilazane at 110℃; for 12h; Inert atmosphere; Sealed tube; | 78% |

| Conditions | Yield |
|---|---|
| With aluminium trichloride In carbon disulfide for 3h; Ambient temperature; | 77% |

| Conditions | Yield |
|---|---|
| With 1-methyl-pyrrolidin-2-one; sodium hydride at 100℃; for 15h; | 77% |
-
-
352-70-5
m-Fluorotoluene

-
-
73183-34-3
bis(pinacol)diborane

-
-
253342-48-2
4,4,5,5-tetramethyl-2-m-tolyl-1,3,2-dioxaborolane

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); lithium hexamethyldisilazane In toluene at 80℃; for 12h; Inert atmosphere; Schlenk technique; | 77% |

| Conditions | Yield |
|---|---|
| With N,N'-difluoro-1,4-diazoniabicyclo<2.2.2>octane bis(tetrafluoroborate); trifluoroacetic acid In acetonitrile at 20℃; for 24h; Irradiation; | 77% |

| Conditions | Yield |
|---|---|
| With aluminium trichloride In carbon disulfide | 76.4% |
| With aluminium trichloride |
-
-
352-70-5
m-Fluorotoluene

-
-
67734-35-4
(R)-tert-butyl tert-butanethiosulfinate

-
-
1361250-93-2
(S)-tert-butyl (3-fluorophenyl)methyl sulfoxide

| Conditions | Yield |
|---|---|
| Stage #1: m-Fluorotoluene With 2,2,6,6-tetramethyl-piperidine; n-butyllithium; potassium tert-butylate In tetrahydrofuran; hexane at -78℃; for 0.583333h; Inert atmosphere; Stage #2: (R)-tert-butyl tert-butanethiosulfinate In tetrahydrofuran; hexane Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 76% |
3-Fluorotoluene Chemical Properties
Molecular Structure of 3-Fluorotoluene (CAS NO.352-70-5):
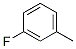
IUPAC Name: 1-Fluoro-3-methylbenzene
Molecular Formula: C7H7F
Molecular Weight: 110.13 g/mol
Density: 1.001 g/cm3
Melting Point: -87 °C
Boiling Point: 118.2 °C at 760 mmHg
Flash Point: 9.4 °C
Water Solubility: immiscible
Index of Refraction: 1.477
Molar Refractivity: 31.07 cm3
Molar Volume: 109.9 cm3
Surface Tension: 27.6 dyne/cm
Enthalpy of Vaporization: 34.16 kJ/mol
Vapour Pressure: 20.1 mmHg at 25 °C
XLogP3: 2.7
H-Bond Acceptor: 1
Exact Mass: 110.053178
MonoIsotopic Mass: 110.053178
Canonical SMILES: CC1=CC(=CC=C1)F
InChI: InChI=1S/C7H7F/c1-6-3-2-4-7(8)5-6/h2-5H,1H3
InChIKey: BTQZKHUEUDPRST-UHFFFAOYSA-N
EINECS: 206-524-8
Product Categories: Aromatic Hydrocarbons (substituted) & Derivatives; Aryl; C7; Halogenated Hydrocarbons
3-Fluorotoluene Uses
3-Fluorotoluene (CAS NO.352-70-5) is used as intermediates of medicine and pesticide.
3-Fluorotoluene Safety Profile
Hazard Codes:  F,
F,  Xi
Xi
Risk Statements: 11-36/37/38
R11:Highly flammable.
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 16-26-36-37/39
S16:Keep away from sources of ignition.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S37/39:Wear suitable gloves and eye/face protection.
RIDADR: UN 2388 3/PG 2
WGK Germany: 2
RTECS: XT2578000
Hazard Note: Flammable/Irritant
TSCA: T
HazardClass: 3
PackingGroup: II
HS Code: 29036990
3-Fluorotoluene Specification
3-Fluorotoluene (CAS NO.352-70-5) is also named as 1-Fluoro-3-methylbenzene ; 1-Methyl-3-fluorobenzene ; Benzene, 1-fluoro-3-methyl- ; NSC 8860 ; m-Fluorotoluene . 3-Fluorotoluene (CAS NO.352-70-5) is clear colorless to light yellow liquid with an aromatic odor. It is highly flammable. 3-Fluorotoluene may be incompatible with strong oxidizing and reducing agents. It is also may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. 3-Fluorotoluene generates toxic fluoride fumes when burned.Inhalation causes upper respiratory irritation. Irritating to skin and eyes. It may be absorbed through the skin. Prolonged exposure may result in systemic toxic effects. It is harmful if swallowed.
Related Products
- 3-Fluorotoluene
- 35271-74-0
- 35272-15-2
- 35272-19-6
- 35274-05-6
- 35275-62-8
- 35276-81-4
- 35276-83-6
- 35279-80-2
- 35285-26-8
- 35285-68-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View