-
Name
4-(4-AMINOPHENYL)MORPHOLIN-3-ONE
- EINECS 610-147-8
- CAS No. 438056-69-0
- Article Data52
- CAS DataBase
- Density 1.268 g/cm3
- Solubility 16.6g/L at 20℃
- Melting Point 171.0 to 175.0 °C
- Formula C10H12N2O2
- Boiling Point 502.3 °C at 760 mmHg
- Molecular Weight 192.217
- Flash Point 257.6 °C
- Transport Information
- Appearance
- Safety 24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 4-(3-Oxo-4-morpholinyl)aniline;4-(4-Aminophenyl)-3-morpholinone;
- PSA 55.56000
- LogP 1.27820
Synthetic route
-
-
446292-04-2
4-(4-nitrophenyl)-3-morpholinone

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen In ethanol at 60℃; under 3000.3 Torr; for 3h; Autoclave; | 97.8% |
| With hydrogen In water; isopropyl alcohol at 50 - 55℃; under 7600.51 - 11400.8 Torr; for 5h; Temperature; Reagent/catalyst; Solvent; Pressure; Autoclave; | 96.4% |
| With hydrogen; palladium on activated charcoal In methanol; water at 40℃; under 1500.15 Torr; for 17h; | 95% |

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In dichloromethane at 0 - 20℃; for 2h; | 96% |
-
-
141-43-5
ethanolamine

-
-
446292-04-2
4-(4-nitrophenyl)-3-morpholinone

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| With iron; acetic acid In acetonitrile at 30℃; for 3h; | 95% |
-
-
446292-04-2
4-(4-nitrophenyl)-3-morpholinone

-
B
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| With iron(III) chloride; pyrographite; hydrazine hydrate In ethanol; water for 2h; Reflux; | A n/a B 93% |

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; potassium carbonate In dichloromethane at 0 - 20℃; for 5h; Concentration; | 92.4% |
| With tetrabutylammomium bromide; potassium carbonate In dichloromethane at 0 - 20℃; for 3h; | 92.4% |
| With tetrabutylammomium bromide; potassium carbonate In dichloromethane at 0 - 20℃; for 5h; Concentration; | 35.2 g |

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water for 2h; Reflux; | 92% |
-
-
1313613-18-1
[4-(3-oxo-morpholin-4-yl)phenyl]carbamic acid benzyl ester

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water for 3h; Reflux; | 90% |

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| With ethanol; 5%-palladium/activated carbon; hydrogen at 40℃; under 2068.65 Torr; for 2h; Inert atmosphere; Heating; | 89.5% |
-
-
1252018-07-7
methyl N-[4-(3-oxo-4-morpholinyl)phenyl]carbamate

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water for 4h; Reflux; | 85% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; copper(l) iodide; N,N`-dimethylethylenediamine In toluene at 110℃; for 2h; Ullmann Condensation; Microwave irradiation; | 75% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; 8-quinolinol; potassium carbonate In dimethyl sulfoxide at 130℃; for 12h; Inert atmosphere; | 53.45% |
| With copper(l) iodide; N,N-dimethyl-formamide at 120℃; Inert atmosphere; | 50% |
| With potassium carbonate; N,N`-dimethylethylenediamine; copper(l) iodide In 1,4-dioxane at 110℃; | |
| With potassium carbonate; copper(l) iodide; N,N`-dimethylethylenediamine In 1,4-dioxane at 110℃; | |
| With copper(l) iodide; potassium carbonate; N,N`-dimethylethylenediamine In 1,4-dioxane at 110℃; |
-
-
100-01-6
4-nitro-aniline

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: toluene / 5 h / 95 °C 2: K2CO3 / acetonitrile / 8 h / 20 °C 3: H2 / Pd/C / methanol / 1 h / 35 °C / 750.06 Torr View Scheme |
-
-
811450-82-5
4-(2-(2-chloroethoxy)acetamido)1-nitrobenzene

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: K2CO3 / acetonitrile / 8 h / 20 °C 2: H2 / Pd/C / methanol / 1 h / 35 °C / 750.06 Torr View Scheme |

-
-
446292-04-2
4-(4-nitrophenyl)-3-morpholinone

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| In ethanol; ethyl acetate |
-
-
122-98-5
2-Anilinoethanol

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sodium hydroxide / ethanol; water / 0.5 h / 38 - 45 °C / pH 10 - 13 / Industry scale 2: nitric acid; sulfuric acid / sulfolane / 2 h / -5 - 30 °C 3: hydrogen / Raney Ni / methanol / 24 h / 40 - 50 °C View Scheme | |
| Multi-step reaction with 3 steps 1: sodium hydroxide / isopropyl alcohol / 0 - 40 °C / pH 7 - 8 2: nitric acid; sulfuric acid / 1 h / -10 °C 3: hydrogen / 5% Pd(II)/C(eggshell) / water; tetrahydrofuran / 6.5 h / 70 °C / 2250.23 Torr View Scheme | |
| Multi-step reaction with 3 steps 1: sodium hydroxide / ethanol; water 2: sulfuric acid; nitric acid / water 3: 5%-palladium/activated carbon; hydrogen / ethanol / 40 °C View Scheme |
-
-
29518-11-4
4-phenyl-3-morpholinone

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: nitric acid; sulfuric acid / sulfolane / 2 h / -5 - 30 °C 2: hydrogen / Raney Ni / methanol / 24 h / 40 - 50 °C View Scheme | |
| Multi-step reaction with 2 steps 1: nitric acid; sulfuric acid / 1 h / -10 °C 2: hydrogen / 5% Pd(II)/C(eggshell) / water; tetrahydrofuran / 6.5 h / 70 °C / 2250.23 Torr View Scheme | |
| Multi-step reaction with 2 steps 1: sulfuric acid; nitric acid / water 2: 5%-palladium/activated carbon; hydrogen / ethanol / 40 °C View Scheme |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium carbonate; N,N`-dimethylethylenediamine In toluene Inert atmosphere; Reflux; | |
| With copper(l) iodide; potassium carbonate; N,N`-dimethylethylenediamine In toluene Inert atmosphere; |
-
-
108-86-1
bromobenzene

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: copper(l) iodide; potassium carbonate / water / 15 h / 80 °C 2.1: hydrogenchloride; sodium nitrite / water / 5 h / 0 - 5 °C / Cooling 3.1: triethylamine / tetrahydrofuran / 4.6 h / Cooling with ice 3.2: 1.3 h / 35 °C / Heating 4.1: ethanol; 5%-palladium/activated carbon; hydrogen / 2 h / 40 °C / 2068.65 Torr / Inert atmosphere; Heating View Scheme |
-
-
52671-43-9
4-nitroso-N-2-hydroxyethylaniline

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: triethylamine / tetrahydrofuran / 4.6 h / Cooling with ice 1.2: 1.3 h / 35 °C / Heating 2.1: ethanol; 5%-palladium/activated carbon; hydrogen / 2 h / 40 °C / 2068.65 Torr / Inert atmosphere; Heating View Scheme |
-
-
586-78-7
para-nitrophenyl bromide

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: potassium carbonate / acetonitrile / 18 h / 60 °C 1.2: 2 h / 60 °C 2.1: iron; acetic acid / acetonitrile / 3 h / 30 °C View Scheme |
-
-
100-00-5
4-chlorobenzonitrile

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: potassium carbonate / acetonitrile / 6 h / 60 °C 1.2: 2 h / 60 °C 2.1: iron; acetic acid / acetonitrile / 3 h / 30 °C View Scheme | |
| Multi-step reaction with 3 steps 1: potassium carbonate / 3 h / 100 - 105 °C / Green chemistry 2: hydrogen / water; isopropyl alcohol / 4 h / 50 - 55 °C / 2280.15 - 3040.2 Torr / Autoclave; Green chemistry 3: 5,5-dimethyl-1,3-cyclohexadiene / 5 h / 100 - 110 °C / Green chemistry View Scheme |
-
-
350-46-9
4-Fluoronitrobenzene

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: potassium carbonate / acetonitrile / 18 h / 60 °C 1.2: 2 h / 60 °C 2.1: iron; acetic acid / acetonitrile / 3 h / 30 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: caesium carbonate / acetonitrile / 0.5 h / 10 - 20 °C / Inert atmosphere 1.2: 5 h / 20 - 80 °C 2.1: caesium carbonate / 1,4-dioxane / Reflux 3.1: potassium tert-butylate / toluene / 5 h / 100 °C 4.1: palladium 10% on activated carbon; hydrogen / methanol / 10 h / 70 °C / 2280.15 Torr View Scheme | |
| Multi-step reaction with 4 steps 1.1: potassium carbonate / acetonitrile / 0.83 h / 10 - 20 °C / Inert atmosphere 1.2: 10 h / 20 - 35 °C 2.1: potassium carbonate / 1,4-dioxane / Reflux 3.1: caesium carbonate / toluene / 10 h / 70 °C 4.1: palladium 10% on activated carbon; hydrogen / methanol / 10 h / 70 °C / 2280.15 Torr View Scheme |

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: potassium carbonate / N,N-dimethyl-formamide / 8 h / 100 °C 2.1: acetic anhydride; nitric acid / 5 h / 0 - 25 °C 3.1: iron(III) chloride hexahydrate; pyrographite / methanol / 0.5 h / Reflux 3.2: 5 h / Cooling; Reflux View Scheme |
-
-
103-84-4
Acetanilid

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: nitric acid; acetic acid; sulfuric acid / 2 h / 0 - 25 °C 2.1: potassium carbonate / tetrahydrofuran / 4 h / 0 - 55 °C 3.1: potassium carbonate / acetonitrile / 6 h / Reflux 4.1: acetic anhydride; nitric acid / 5 h / 0 - 25 °C 5.1: iron(III) chloride hexahydrate; pyrographite / methanol / 0.5 h / Reflux 5.2: 5 h / Cooling; Reflux View Scheme |
-
-
100-01-6
4-nitro-aniline

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: potassium carbonate / tetrahydrofuran / 4 h / 0 - 55 °C 2.1: potassium carbonate / acetonitrile / 6 h / Reflux 3.1: acetic anhydride; nitric acid / 5 h / 0 - 25 °C 4.1: iron(III) chloride hexahydrate; pyrographite / methanol / 0.5 h / Reflux 4.2: 5 h / Cooling; Reflux View Scheme | |
| Multi-step reaction with 3 steps 1: toluene / 6.5 h / 5 - 98 °C 2: potassium carbonate / acetonitrile / 10 h / 20 °C 3: 5%-palladium/activated carbon; hydrogen / methanol View Scheme |

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: potassium carbonate / acetonitrile / 6 h / Reflux 2.1: acetic anhydride; nitric acid / 5 h / 0 - 25 °C 3.1: iron(III) chloride hexahydrate; pyrographite / methanol / 0.5 h / Reflux 3.2: 5 h / Cooling; Reflux View Scheme |
-
-
62-53-3
aniline

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: triethylamine / dichloromethane / 4 h / 0 - 25 °C 2.1: potassium carbonate / acetonitrile / 6 h / Reflux 3.1: acetic anhydride; nitric acid / 5 h / 0 - 25 °C 4.1: iron(III) chloride hexahydrate; pyrographite / methanol / 0.5 h / Reflux 4.2: 5 h / Cooling; Reflux View Scheme | |
| Multi-step reaction with 4 steps 1.1: triethylamine / dichloromethane / 4 h / 0 - 5 °C 2.1: potassium carbonate / N,N-dimethyl-formamide / 8 h / 100 °C 3.1: acetic anhydride; nitric acid / 5 h / 0 - 25 °C 4.1: iron(III) chloride hexahydrate; pyrographite / methanol / 0.5 h / Reflux 4.2: 5 h / Cooling; Reflux View Scheme | |
| Multi-step reaction with 4 steps 1.1: triethylamine / tert-butyl methyl ether / 4 h / 0 - 5 °C 2.1: potassium carbonate / N,N-dimethyl-formamide / 8 h / 100 °C 3.1: acetic anhydride; nitric acid / 5 h / 0 - 25 °C 4.1: iron(III) chloride hexahydrate; pyrographite / methanol / 0.5 h / Reflux 4.2: 5 h / Cooling; Reflux View Scheme |

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: potassium carbonate / N,N-dimethyl-formamide / 8 h / 100 °C 2.1: acetic anhydride; nitric acid / 5 h / 0 - 25 °C 3.1: iron(III) chloride hexahydrate; pyrographite / methanol / 0.5 h / Reflux 3.2: 5 h / Cooling; Reflux View Scheme |
-
-
106-50-3
1,4-phenylenediamine

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine / tetrahydrofuran / 5 h / 10 - 20 °C 2: tetrabutylammomium bromide; potassium carbonate / dichloromethane / 5 h / 0 - 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: pyridine / tetrahydrofuran / 3 h / 10 - 20 °C 2: potassium carbonate; tetrabutylammomium bromide / dichloromethane / 3 h / 0 - 20 °C View Scheme |
-
-
57044-25-4
(R)-oxiranemethanol

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

-
-
1447919-65-4
4-[4-{(R)-2,3-dihydroxy-propylamino}phenyl]morpholin-3-one

| Conditions | Yield |
|---|---|
| In methanol for 14h; Reflux; | 100% |
-
-
501-53-1
benzyl chloroformate

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

-
-
1313613-18-1
[4-(3-oxo-morpholin-4-yl)phenyl]carbamic acid benzyl ester

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water; acetone; toluene at 0 - 30℃; for 4h; | 98% |
| With sodium hydrogencarbonate In water; acetone; toluene at 0 - 20℃; for 4h; | 98% |
| With sodium hydrogencarbonate In water; acetone at 0℃; for 3.5h; | 96.2% |

| Conditions | Yield |
|---|---|
| With pyridine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 6h; Inert atmosphere; | 98% |
-
-
42518-98-9
5-chlorothiophene-2-carbonyl chloride

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 5h; | 97.5% |

| Conditions | Yield |
|---|---|
| With pyridine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 6h; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With pyridine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 6h; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| In ethanol at 36℃; for 7h; Solvent; | 96.4% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water at 0 - 10℃; | 96.1% |
-
-
161596-47-0
(S)-N-glycidylphthalimide

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

-
-
446292-07-5
2-((2R)-2-hydroxy-3-{[4-(3-oxo-4-morpholinyl)phenyl]amino}propyl)-1H-isoindole-1,3(2H)-dione

| Conditions | Yield |
|---|---|
| In ethanol; water at 20℃; for 15h; Solvent; Temperature; | 95% |
| In ethanol; water for 27h; Heating; | 92% |
| In methanol; water at 65 - 70℃; for 32h; | 92% |
-
-
51594-55-9
(R)-(-)-epichlorohydrin

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

-
-
1252018-10-2
4-[4-(N-(3-chloro-(2R)-2-hydroxy-1-propyl)amino)phenyl]morpholin-3-one

| Conditions | Yield |
|---|---|
| In ethanol for 12h; Reflux; | 95% |
| In water; isopropyl alcohol at 25 - 35℃; for 17h; Solvent; Time; | 90% |
| In isopropyl alcohol for 12h; Reflux; | 90% |
-
-
79-22-1
methyl chloroformate

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

-
-
1252018-07-7
methyl N-[4-(3-oxo-4-morpholinyl)phenyl]carbamate

| Conditions | Yield |
|---|---|
| Stage #1: methyl chloroformate; 4-(4-aminophenyl)morpholin-3-one With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 0.3h; Stage #2: methyl chloroformate In dichloromethane for 1.16667h; | 95% |

| Conditions | Yield |
|---|---|
| With pyridine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 6h; Inert atmosphere; | 95% |
-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| With C40H51ClIrN3; potassium carbonate; deuterium In dichloromethane at 50℃; under 760.051 Torr; for 12h; Sealed tube; Inert atmosphere; regioselective reaction; | 95% |
-
-
103723-94-0
3-methylbut-2-enyl chloroformate

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 10℃; | 94.8% |
-
-
161596-47-0
(S)-N-glycidylphthalimide

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| In water; toluene at 30 - 85℃; Solvent; Temperature; | 94.6% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| Stage #1: 4-(4-aminophenyl)morpholin-3-one With triethylamine In dichloromethane at 20℃; for 0.25h; Stage #2: di-tert-butyl dicarbonate In dichloromethane at 20℃; for 3h; | 94.2% |
-
-
24460-74-0
n-dodecyl chloroformate

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

-
-
1414932-71-0
dodecyl (4-(3-oxomorpholin)phenyl)carbamate

| Conditions | Yield |
|---|---|
| Stage #1: 4-(4-aminophenyl)morpholin-3-one With sodium carbonate In water; acetone at 0℃; for 0.166667h; Stage #2: n-dodecyl chloroformate at 20℃; for 4h; | 94% |
| Stage #1: 4-(4-aminophenyl)morpholin-3-one With sodium carbonate In water; acetone at 0℃; for 0.166667h; Stage #2: n-dodecyl chloroformate In water; acetone at 20℃; for 4h; | 94% |
-
-
41994-44-9
5-chloro-2-nitrobenzoyl chloride

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| With dmap; potassium carbonate In tetrahydrofuran for 2h; Reflux; | 94% |
-
-
51673-84-8
dimethoxyacetaldehyde

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| Stage #1: dimethoxyacetaldehyde; 4-(4-aminophenyl)morpholin-3-one In dichloromethane; water at 20℃; for 2h; Molecular sieve; Stage #2: With sodium tris(acetoxy)borohydride In dichloromethane; water at 20℃; for 1h; | 94% |

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane Reflux; | 93.7% |
-
-
135042-12-5
(4R)-1-(tert-butoxycarbonyl)-4-hydroxy-D-proline

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

-
-
773889-47-7
(2R,4R)-4-hydroxy-2-[4-(3-oxo-morpholin-4-yl)-phenylcarbamoyl]-pyrrolidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline In toluene at 20℃; for 18h; | 93.2% |
-
-
610-14-0
2-nitrobenzyl chloride

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| With dmap; potassium carbonate In tetrahydrofuran for 2h; Reflux; | 93% |
-
-
50-00-0
formaldehyd

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

| Conditions | Yield |
|---|---|
| In dichloromethane at 20 - 30℃; for 2h; Time; | 92.1% |
| In dichloromethane at 20 - 30℃; for 2h; Time; | 92.1% |
| In dichloromethane at 20 - 30℃; for 5h; Concentration; | 92.1% |
| In dichloromethane at 20 - 30℃; for 5h; Solvent; | 185.4 g |
| In dichloromethane at 20 - 30℃; for 2h; Time; | 188.1 g |
-
-
348626-26-6
5-chloro-N-(oxiran-2-ylmethyl)thiophene-2-carboxamide

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

-
-
721401-53-2
5-chloro-N-{(R)‐2‐hydroxy‐3‐[4‐(3-oxo-4-morpholinyl)phenylamino]-propyl}thiophene-2-carboxamide

| Conditions | Yield |
|---|---|
| ytterbium(III) triflate In tetrahydrofuran at 20 - 60℃; | 92% |
| With magnesium(II) perchlorate In acetone at 50℃; for 18h; Inert atmosphere; | 76% |
-
-
1412905-05-5
2-[(2-oxo-2H-chromen-4-yl)oxy]propanoic acid

-
-
438056-69-0
4-(4-aminophenyl)morpholin-3-one

-
-
1412905-15-7
2-(2-oxo-2H-chromen-4-yloxy)-N-(4-(3-oxomorpholino)phenyl)propanamide

| Conditions | Yield |
|---|---|
| With HATU In N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 92% |
4-(4-Aminophenyl)morpholin-3-one Chemical Properties
Following is the structure of 4-(4-Aminophenyl)morpholin-3-one (CAS NO.438056-69-0):
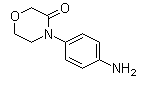
Empirical Formula: C10H12N2O2
Molecular Weight: 192.2145
Molar Refractivity: 52.42 cm3
Molar Volume: 151.5 cm3
Density: 1.268 g/cm3
Flash Point: 257.6 °C
Index of Refraction: 1.608
Surface Tension: 55.8 dyne/cm
Enthalpy of Vaporization: 77.12 kJ/mol
Boiling Point: 502.3 °C at 760 mmHg
Vapour Pressure of 4-(4-Aminophenyl)morpholin-3-one (CAS NO.438056-69-0): 3.23E-10 mmHg at 25 °C
Product Categories of 4-(4-Aminophenyl)morpholin-3-one (CAS NO.438056-69-0): Amines and Anilines; Heterocycles
SMILES: O=C2N(c1ccc(N)cc1)CCOC2
InChI: InChI=1/C10H12N2O2/c11-8-1-3-9(4-2-8)12-5-6-14-7-10(12)13/h1-4H,5-7,11H2
InChIKey: MHCRLDZZHOVFEE-UHFFFAOYAC
4-(4-Aminophenyl)morpholin-3-one Specification
4-(4-Aminophenyl)morpholin-3-one , its cas register number is 438056-69-0. It also can be called 3-Morpholinone, 4-(4-aminophenyl)- .
Related Products
- 4-(4-Aminophenyl)morpholin-3-one
- 4380-96-5
- 4381-25-3
- 438190-90-0
- 438219-92-2
- 438-23-3
- 438234-34-5
- 438237-86-6
- 438249-83-3
- 438249-89-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View