-
Name
4-Chloro-6,7-bis(2-methoxyethoxy)quinazoline
- EINECS 605-994-5
- CAS No. 183322-18-1
- Article Data51
- CAS DataBase
- Density 1.257 g/cm3
- Solubility
- Melting Point 105-107 °C
- Formula C14H17ClN2O4
- Boiling Point 446.7 °C at 760 mmHg
- Molecular Weight 312.753
- Flash Point 224 °C
- Transport Information
- Appearance
- Safety 26-39
- Risk Codes 22-41
-
Molecular Structure
- Hazard Symbols Xn
- Synonyms 4-Chloro-6,7-bis-(1-methoxy-ethoxy)-quinazoline;4-Chloro-6,7-(2-methoxyethoxy)-quinazoline;4-chloro-6,7-bis(2-methoxyethoxy)-4(3H)-quinazoline;
- PSA 62.70000
- LogP 2.33360
Synthetic route
-
-
179688-29-0
6,7-bis(2-methoxyethoxy)quinazolin-4(3H)-one

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; trichlorophosphate In toluene Heating; | 100% |
| With triethylamine; trichlorophosphate In toluene at 70 - 80℃; for 3h; | 90% |
| Stage #1: 6,7-bis-(2-methoxyethoxy)-4(3H)quinazolinone With thionyl chloride; N,N-dimethyl-formamide In dichloromethane for 6h; Reflux; Stage #2: With sodium hydroxide In dichloromethane at 20℃; for 0.5h; pH=7 - 8; | 89.5% |
| With thionyl chloride; N,N-dimethyl-formamide In dichloromethane for 6h; Reflux; | 89.5% |

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| With oxalyl dichloride In dichloromethane; N,N-dimethyl-formamide at 50℃; for 3h; | 99.3% |
-
-
179688-29-0
6,7-bis(2-methoxyethoxy)-3,4-dihydroquinazolin-4-one

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| With oxalyl dichloride In chloroform; N,N-dimethyl-formamide for 1.5h; Heating; | 98% |
| With oxalyl dichloride In dichloromethane Solvent; Reagent/catalyst; Temperature; Reflux; Large scale; | 97% |
| With thionyl chloride In N,N-dimethyl-formamide at 60℃; Temperature; Concentration; | 95.6% |
-
-
179688-29-0
C14H18N2O5

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| With trichlorophosphate In dichloromethane; N,N-dimethyl-formamide at 20℃; for 6h; Reagent/catalyst; | 97.1% |
| With thionyl chloride In dichloromethane; N,N-dimethyl-formamide at 30℃; for 5 - 13h; Product distribution / selectivity; Heating / reflux; | |
| Stage #1: C14H18N2O5 With thionyl chloride In dichloromethane; N,N-dimethyl-formamide at 30℃; for 5h; Heating / reflux; Stage #2: With sodium hydroxide In dichloromethane; water; N,N-dimethyl-formamide at 25℃; for 0.25 - 0.333333h; pH=7 - 8; | |
| Stage #1: C14H18N2O5 With thionyl chloride In dichloromethane; N,N-dimethyl-formamide at 30℃; for 5h; Heating / reflux; Stage #2: With sodium hydroxide In dichloromethane; water; N,N-dimethyl-formamide at 25℃; for 0.25 - 0.333333h; pH=7 - 8; | |
| With thionyl chloride; N,N-dimethyl-formamide In dichloromethane for 5 - 13h; Product distribution / selectivity; Heating / reflux; |

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| 92% | |
| 92% | |
| With oxalyl dichloride; N,N-dimethyl-formamide In methanol for 1.5h; Reflux; | 92% |
| With oxalyl dichloride In chloroform; N,N-dimethyl-formamide for 1.5h; Heating / reflux; |
-
-
1006377-63-4
C13H19NO5

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: acetic anhydride / 110 °C 2.1: 85 percent / aq. nitric acid / acetic acid / 8 h / 45 - 50 °C 3.1: FeCl3; hydrazine hydrate / H2O; methanol / 3 h / Heating 3.2: 81 percent / HCl / H2O / 90 - 130 °C 4.1: 94 percent / thionyl chloride / dimethylformamide / 2 h / Heating View Scheme |
-
-
80407-68-7
3,4-bis(2-methoxyethoxy) benzonitrile

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 85 percent / aq. nitric acid / acetic acid / 8 h / 45 - 50 °C 2.1: FeCl3; hydrazine hydrate / H2O; methanol / 3 h / Heating 2.2: 81 percent / HCl / H2O / 90 - 130 °C 3.1: 94 percent / thionyl chloride / dimethylformamide / 2 h / Heating View Scheme | |
| Multi-step reaction with 5 steps 1: nitric acid 2: dihydrogen peroxide 3: palladium on activated charcoal; ammonium formate 4: water / 24 h / 130 °C 5: trichlorophosphate; triethylamine View Scheme |
-
-
236750-65-5
2-nitro-4,5-bis(2-methoxyethoxy)benzonitrile

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: FeCl3; hydrazine hydrate / H2O; methanol / 3 h / Heating 1.2: 81 percent / HCl / H2O / 90 - 130 °C 2.1: 94 percent / thionyl chloride / dimethylformamide / 2 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: hydrazine hydrate / water / 3 h / 20 - 30 °C 2: trifluoroacetic acid / ethyl acetate / 4 h / Reflux 3: trichlorophosphate; N,N-dimethyl-formamide / ethyl acetate / 2 h / 70 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 4 steps 1: dihydrogen peroxide 2: palladium on activated charcoal; ammonium formate 3: water / 24 h / 130 °C 4: trichlorophosphate; triethylamine View Scheme |
-
-
80407-64-3
3,4-bis(2-methoxyethoxy)benzaldehyde

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: hydroxylamine hydrochloride; pyridine / methanol / Heating 2.1: acetic anhydride / 110 °C 3.1: 85 percent / aq. nitric acid / acetic acid / 8 h / 45 - 50 °C 4.1: FeCl3; hydrazine hydrate / H2O; methanol / 3 h / Heating 4.2: 81 percent / HCl / H2O / 90 - 130 °C 5.1: 94 percent / thionyl chloride / dimethylformamide / 2 h / Heating View Scheme | |
| Multi-step reaction with 6 steps 1: hydroxylamine hydrochloride; acetic anhydride 2: nitric acid 3: dihydrogen peroxide 4: palladium on activated charcoal; ammonium formate 5: water / 24 h / 130 °C 6: trichlorophosphate; triethylamine View Scheme |
-
-
139-85-5
3,4-dihydroxybenzaldehyde

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: 98 percent / potassium carbonate / dimethylformamide / 100 °C 2.1: hydroxylamine hydrochloride; pyridine / methanol / Heating 3.1: acetic anhydride / 110 °C 4.1: 85 percent / aq. nitric acid / acetic acid / 8 h / 45 - 50 °C 5.1: FeCl3; hydrazine hydrate / H2O; methanol / 3 h / Heating 5.2: 81 percent / HCl / H2O / 90 - 130 °C 6.1: 94 percent / thionyl chloride / dimethylformamide / 2 h / Heating View Scheme |
-
-
3943-89-3
Ethyl protocatechuate

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: potassium carbonate; tetrabutylammonium iodide / acetone / 120 h / Heating 2: acetic acid; nitric acid / 24 h / 20 °C 3: hydrogen / PtO2*H2O / methanol / 20 °C / 2585.74 Torr 4: 84 percent / 12 h / 165 - 170 °C 5: phosphoryl choride; N,N-diethylaniline / 0.67 h / 70 - 90 °C View Scheme | |
| Multi-step reaction with 5 steps 1: potassium carbonate; tetrabutylammonium iodide / acetone 2: acetic acid; nitric acid / 24 h / 20 °C 3: hydrogen / PtO2*H2O / methanol / 20 °C / 2585.74 Torr 4: 84 percent / 12 h / 165 - 170 °C 5: phosphoryl choride; N,N-diethylaniline / 0.67 h / 70 - 90 °C View Scheme | |
| Multi-step reaction with 5 steps 1: 68 percent / potassium carbonate; tetrabutylammonium iodide / acetone / 72 h / Heating 2: nitric acid; trifluoroacetic acid / CH2Cl2 / 20 °C 3: 94 percent / hydrogen / palladium on activated carbon / methanol 4: 62 percent / formamide / 3 h / 160 °C 5: 98 percent / oxalyl chloride / CHCl3; dimethylformamide / 1.5 h / Heating View Scheme |
-
-
183322-16-9
3,4-bis(2-methoxyethoxy)benzoic acid ethyl ester

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: acetic acid; nitric acid / 24 h / 20 °C 2: hydrogen / PtO2*H2O / methanol / 20 °C / 2585.74 Torr 3: 84 percent / 12 h / 165 - 170 °C 4: phosphoryl choride; N,N-diethylaniline / 0.67 h / 70 - 90 °C View Scheme | |
| Multi-step reaction with 4 steps 1: nitric acid; trifluoroacetic acid / CH2Cl2 / 20 °C 2: 94 percent / hydrogen / palladium on activated carbon / methanol 3: 62 percent / formamide / 3 h / 160 °C 4: 98 percent / oxalyl chloride / CHCl3; dimethylformamide / 1.5 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1.1: nitric acid / acetic acid / 24 h / 20 °C / cooling with ice-water 2.1: ammonium formate / 5%-palladium/activated carbon / 7 h / 150 °C 3.1: thionyl chloride / N,N-dimethyl-formamide / dichloromethane / 6 h / Reflux 3.2: 0.5 h / 20 °C / pH 7 - 8 View Scheme |
-
-
179688-27-8
ethyl 2-amino-4,5-bis(2-methoxyethoxy)-benzoate

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 84 percent / 12 h / 165 - 170 °C 2: phosphoryl choride; N,N-diethylaniline / 0.67 h / 70 - 90 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 62 percent / formamide / 3 h / 160 °C 2: 98 percent / oxalyl chloride / CHCl3; dimethylformamide / 1.5 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1.1: N,N-dimethyl-formamide / 10 h / 130 - 140 °C 1.2: 65 - 80 °C 2.1: trichlorophosphate; triethylamine / toluene / 3 h / 70 - 80 °C View Scheme |
-
-
179688-26-7
2-nitro-4,5-bis(2-methoxyethoxy)benzoic acid ethyl ester

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: hydrogen / PtO2*H2O / methanol / 20 °C / 2585.74 Torr 2: 84 percent / 12 h / 165 - 170 °C 3: phosphoryl choride; N,N-diethylaniline / 0.67 h / 70 - 90 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 94 percent / hydrogen / palladium on activated carbon / methanol 2: 62 percent / formamide / 3 h / 160 °C 3: 98 percent / oxalyl chloride / CHCl3; dimethylformamide / 1.5 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1.1: ammonium formate / 5%-palladium/activated carbon / 7 h / 150 °C 2.1: thionyl chloride / N,N-dimethyl-formamide / dichloromethane / 6 h / Reflux 2.2: 0.5 h / 20 °C / pH 7 - 8 View Scheme |
-
-
99-50-3
3,4-Dihydroxybenzoic acid

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 98 percent / H2SO4 / Heating 2: 99 percent / K2CO3; TBAI / acetone / Heating 3: 85 percent / HNO3; Ac2O / 20 °C 4: 100 percent / H2 / Pd/C / ethanol 5: 90 percent / 160 °C 6: 100 percent / POCl3; diisopropylethylamine / toluene / Heating View Scheme | |
| Multi-step reaction with 6 steps 1.1: sulfuric acid / 10 h / 40 - 80 °C 2.1: potassium tert-butylate; potassium iodide / N,N-dimethyl-formamide / 12 h / 100 °C 3.1: sulfuric acid; nitric acid / 1 h / 20 °C / Darkness 4.1: palladium 10% on activated carbon / ethanol / 10 h / 20 °C 4.2: 20 °C 5.1: ammonium formate; triethylamine / 6 h / 160 °C 6.1: oxalyl dichloride / N,N-dimethyl-formamide; dichloromethane / 3 h / 50 °C View Scheme |
-
-
2150-43-8
3,4-dihydroxybenzoic acid methyl ester

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 99 percent / K2CO3; TBAI / acetone / Heating 2: 85 percent / HNO3; Ac2O / 20 °C 3: 100 percent / H2 / Pd/C / ethanol 4: 90 percent / 160 °C 5: 100 percent / POCl3; diisopropylethylamine / toluene / Heating View Scheme |
-
-
179688-14-3
methyl 3,4-bis{[2-(methyloxy)ethyl]oxy}benzoate

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 85 percent / HNO3; Ac2O / 20 °C 2: 100 percent / H2 / Pd/C / ethanol 3: 90 percent / 160 °C 4: 100 percent / POCl3; diisopropylethylamine / toluene / Heating View Scheme | |
| Multi-step reaction with 4 steps 1: nitric acid; acetic anhydride; acetic acid / acetic acid / 0 - 5 °C 2: hydrogen / ethanol 3: ethanol / Reflux 4: thionyl chloride / N,N-dimethyl-formamide / Reflux View Scheme |
-
-
476168-17-9
2-amino-4,5-bis-(2-methoxyethoxy)-benzoic acid methyl ester

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 90 percent / 160 °C 2: 100 percent / POCl3; diisopropylethylamine / toluene / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: ethanol / Reflux 2: thionyl chloride / N,N-dimethyl-formamide / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: formamide / 160 - 165 °C 2: thionyl chloride / N,N-dimethyl-formamide / 0.67 h / 85 °C View Scheme |
-
-
501684-22-6
methyl 4,5-bis(2-methoxyethoxy)-2-nitrobenzoate

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 100 percent / H2 / Pd/C / ethanol 2: 90 percent / 160 °C 3: 100 percent / POCl3; diisopropylethylamine / toluene / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: hydrogen / ethanol 2: ethanol / Reflux 3: thionyl chloride / N,N-dimethyl-formamide / Reflux View Scheme |
-
-
109-86-4
2-methoxy-ethanol

-
-
1145671-36-8
4-chloro-6,7-dihydroxyquinazoline

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| With PS-triphenylphosphine; di-tert-butyl-diazodicarboxylate In dichloromethane at 0 - 20℃; Mitsunobu reaction; |

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 25 - 145 °C 2: oxalyl dichloride; N,N-dimethyl-formamide / dichloromethane / 2 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: ammonium formate / 3 h / 160 - 165 °C / Inert atmosphere 2: oxalyl dichloride; N,N-dimethyl-formamide / methanol / 1.5 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: ammonium formate / 3 h / 160 - 165 °C / Inert atmosphere 2: pyridine; trichlorophosphate / 2.5 h / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: formic acid / 160 °C / Large scale 2: oxalyl dichloride / dichloromethane / Reflux; Large scale View Scheme | |
| Multi-step reaction with 2 steps 1: ammonium formate; triethylamine / 6 h / 160 °C 2: oxalyl dichloride / N,N-dimethyl-formamide; dichloromethane / 3 h / 50 °C View Scheme |

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: sodium hydroxide / methanol / 2 h / 20 °C 2: dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; silver hexafluoroantimonate; [bis(acetoxy)iodo]benzene / dichloromethane / 16 h / 40 °C / Inert atmosphere 3: sodium hydroxide / ethanol / 20 °C 4: ethanol / 1 h / Reflux 5: hydrogenchloride / water / 2 h / 100 - 105 °C 6: trichlorophosphate / toluene / 4 h / Reflux View Scheme |
-
-
819813-71-3
3,4-bis(2-methoxyethoxy)-benzoic acid

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: oxalyl dichloride; N,N-dimethyl-formamide / dichloromethane / 3 h / 0 - 20 °C 2: triethylamine / dichloromethane / 0 - 20 °C 3: triethylamine; dmap / dichloromethane / 24 h / 20 °C 4: sodium hydroxide / methanol / 2 h / 20 °C 5: dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; silver hexafluoroantimonate; [bis(acetoxy)iodo]benzene / dichloromethane / 16 h / 40 °C / Inert atmosphere 6: sodium hydroxide / ethanol / 20 °C 7: ethanol / 1 h / Reflux 8: hydrogenchloride / water / 2 h / 100 - 105 °C 9: trichlorophosphate / toluene / 4 h / Reflux View Scheme | |
| Multi-step reaction with 5 steps 1: sulfuric acid / Cooling with ice; Reflux 2: nitric acid; acetic acid / 24.5 h / 0 - 20 °C 3: palladium 10% on activated carbon; hydrogen / methanol / 12 h 4: 12 h / 165 - 170 °C / Inert atmosphere 5: trichlorophosphate / 6 h / Reflux View Scheme |

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: triethylamine / dichloromethane / 0 - 20 °C 2: triethylamine; dmap / dichloromethane / 24 h / 20 °C 3: sodium hydroxide / methanol / 2 h / 20 °C 4: dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; silver hexafluoroantimonate; [bis(acetoxy)iodo]benzene / dichloromethane / 16 h / 40 °C / Inert atmosphere 5: sodium hydroxide / ethanol / 20 °C 6: ethanol / 1 h / Reflux 7: hydrogenchloride / water / 2 h / 100 - 105 °C 8: trichlorophosphate / toluene / 4 h / Reflux View Scheme |

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: triethylamine; dmap / dichloromethane / 24 h / 20 °C 2: sodium hydroxide / methanol / 2 h / 20 °C 3: dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; silver hexafluoroantimonate; [bis(acetoxy)iodo]benzene / dichloromethane / 16 h / 40 °C / Inert atmosphere 4: sodium hydroxide / ethanol / 20 °C 5: ethanol / 1 h / Reflux 6: hydrogenchloride / water / 2 h / 100 - 105 °C 7: trichlorophosphate / toluene / 4 h / Reflux View Scheme |

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; silver hexafluoroantimonate; [bis(acetoxy)iodo]benzene / dichloromethane / 16 h / 40 °C / Inert atmosphere 2: sodium hydroxide / ethanol / 20 °C 3: ethanol / 1 h / Reflux 4: hydrogenchloride / water / 2 h / 100 - 105 °C 5: trichlorophosphate / toluene / 4 h / Reflux View Scheme |

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium hydroxide / ethanol / 20 °C 2: ethanol / 1 h / Reflux 3: hydrogenchloride / water / 2 h / 100 - 105 °C 4: trichlorophosphate / toluene / 4 h / Reflux View Scheme |

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: ethanol / 1 h / Reflux 2: hydrogenchloride / water / 2 h / 100 - 105 °C 3: trichlorophosphate / toluene / 4 h / Reflux View Scheme |

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogenchloride / water / 2 h / 100 - 105 °C 2: trichlorophosphate / toluene / 4 h / Reflux View Scheme |
-
-
540-38-5
4-Iodophenol

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

-
-
882511-67-3
4-(4-iodo-phenoxy)-6,7-bis-(2-methoxy-ethoxy)-quinazoline

| Conditions | Yield |
|---|---|
| With potassium carbonate In isopropyl alcohol Heating; | 100% |
-
-
626-01-7
3-Iodoaniline

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

-
-
882511-64-0
[6,7-bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-(3-iodo-phenyl)-amine

| Conditions | Yield |
|---|---|
| With isopropyl alcohol Heating; | 100% |
-
-
540-37-4
p-aminoiodobenzene

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

-
-
882511-65-1
[6,7-bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-(4-iodo-phenyl)-amine

| Conditions | Yield |
|---|---|
| With isopropyl alcohol Heating; | 100% |
-
-
69088-96-6
2-methyl-4-(3-aminophenyl)-3-butyn-2-ol

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

-
-
299912-59-7
3-butyn-2-ol, 4-[3-[[6,7-bis(2-methoxyethoxy)-4-quinazolinyl]amine]phenyl]-2-methyl, hydrochloride

| Conditions | Yield |
|---|---|
| In acetonitrile at 25℃; for 5h; Inert atmosphere; Reflux; | 100% |
| In acetonitrile for 5h; Inert atmosphere; Reflux; | 100% |
-
-
138227-69-7
1-tert-butyloxycarbonyl-4-(4-amino-2-methylphenyloxy)piperidine

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| Stage #1: 1-tert-butyloxycarbonyl-4-(4-amino-2-methylphenyloxy)piperidine; 4-chloro-6,7-bis(2-methoxyethoxy)quinazoline In 1,2-dichloro-ethane; tert-butyl alcohol at 90℃; for 1h; Stage #2: With hydrogenchloride In 1,2-dichloro-ethane; tert-butyl alcohol at 20℃; for 0.166667h; | 99% |
-
-
54060-30-9
3-acetylenephenylamine

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

-
-
183319-69-9
erlotinib hydrochloride

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 18h; Heating; | 98% |
| In iso-propanol (IPA) for 0.5h; Product distribution / selectivity; Heating / reflux; | 97% |
| Stage #1: 3-acetylenephenylamine; 4-chloro-6,7-bis(2-methoxyethoxy)quinazoline In N,N-dimethyl-formamide; acetonitrile at 90℃; for 7h; Darkness; Stage #2: With hydrogenchloride In water at 15 - 30℃; Temperature; Solvent; | 97.6% |
-
-
875-79-6
1,2-dimethylindole

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol; bis(trifluoromethanesulfonyl)amide at 100℃; for 6h; Sealed tube; | 98% |
-
-
13228-39-2
1-ethyl-2-phenyl-1H-indole

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol; bis(trifluoromethanesulfonyl)amide at 100℃; for 6h; Sealed tube; | 98% |
-
-
104-94-9
4-methoxy-aniline

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

-
-
299912-64-4
6,7-bis(2-methoxyethoxy)-N-(4-methoxyphenyl)quinazolin-4-amine

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 80℃; for 0.666667h; Temperature; | 97.21% |
| In water; isopropyl alcohol |
-
-
54060-30-9
3-acetylenephenylamine

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

-
-
183321-74-6
erlotinib

| Conditions | Yield |
|---|---|
| In isopropyl alcohol Concentration; Reflux; | 96.6% |
| In isopropyl alcohol Temperature; Reflux; | 96.6% |
| In isopropyl alcohol at 20℃; Concentration; Temperature; Reflux; | 96.6% |
-
-
52273-77-5
2-(3-aminophenyl)ethan-1-ol

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| With pyridine In isopropyl alcohol for 4h; Reflux; Inert atmosphere; | 96% |
-
-
125872-97-1
ethyl (E)-3-(3-aminophenyl)acrylate

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

-
-
1453173-53-9
ethyl (E)-3-(3-((6,7-bis(2-methoxyethoxy)quinazolin-4-yl)amino)phenyl)acrylate

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 5h; Reflux; | 93.6% |
-
-
2243-47-2
3-biphenyl amine

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

-
-
1456819-76-3
[6,7-di(2-methoxyethoxy)quinazolin-4-yl]-(biphenyl-3'-yl)amine hydrochloride

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 80℃; for 0.25h; Microwave irradiation; | 93% |
-
-
587-02-0
m-ethylaniline

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

-
-
299912-61-1
N-(3-ethylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine

| Conditions | Yield |
|---|---|
| In water; ethyl acetate; isopropyl alcohol | 90% |

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 2h; Reflux; | 89% |
-
-
861106-92-5
(2-amino-6-bromophenyl)methanol

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

-
-
1347720-73-3
C21H24BrN3O5

| Conditions | Yield |
|---|---|
| With pyridine In acetonitrile for 4h; Reflux; | 88.89% |
| With pyridine In acetonitrile for 4h; Reflux; | 88.89% |
-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

-
-
299912-58-6
6,7-bis(2-methoxyethoxy)-N-[3-[(trimethylsilyl)ethynyl]phenyl]-4-quinazolinamine, monohydrochloride

| Conditions | Yield |
|---|---|
| With aniline In isopropyl alcohol | 88% |
-
-
39885-08-0
(2-amino-6-chlorophenyl)methanol

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

-
-
1347720-70-0
C21H24ClN3O5

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 4h; Reflux; | 87.63% |
| In isopropyl alcohol for 4h; Reflux; | 87.63% |
-
-
1326566-41-9
methyl 3-amino-1H-pyrrole-2-carboxylate

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

-
-
1326566-33-9
methyl 3-(6,7-bis(2-methoxyethoxy)quinazolin-4-ylamino)-1H-pyrrole-2-carboxylate

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 4h; Reflux; | 87% |
-
-
1347720-50-6
1-(2-amino-4-chlorophenyl)pentyl-1-alcohol

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

-
-
1347721-31-6
C25H32ClN3O5

| Conditions | Yield |
|---|---|
| With sulfuric acid In isopropyl alcohol at 20℃; | 86.67% |
-
-
98451-51-5
2-amino-6-nitrobenzyl alcohol

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

-
-
1347720-82-4
C21H24N4O7

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane; water at 20℃; for 7h; | 86.46% |
| With hydrogenchloride In 1,4-dioxane; water at 20℃; for 7h; | 86.46% |
-
-
1347720-24-4
2-amino-6-cyanobenzyl alcohol

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

-
-
1347720-85-7
C22H24N4O5

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In isopropyl alcohol at 20℃; | 86.3% |
-
-
177531-95-2
(2-amino-6-methoxyphenyl)methanol

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

-
-
1347720-91-5
C22H27N3O6

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; | 86.24% |
-
-
603-76-9
1-methylindole

-
-
183322-18-1
4-chloro-6,7-bis(2-methoxyethoxy)quinazoline

| Conditions | Yield |
|---|---|
| With 1,1,1,3',3',3'-hexafluoro-propanol; bis(trifluoromethanesulfonyl)amide at 100℃; for 6h; Sealed tube; | 86% |
4-Chloro-6,7-bis(2-methoxyethoxy)quinazoline Chemical Properties
Empirical Formula: C14H17ClN2O4
Molecular Weight: 312.7488
Nominal Mass: 312 Da
Average Mass: 312.7488 Da
Monoisotopic Mass: 312.087685 Da
Index of Refraction: 1.561
Molar Refractivity: 80.55 cm3
Molar Volume: 248.7 cm3
Surface Tension: 45.8 dyne/cm
Density: 1.257 g/cm3
Flash Point: 224 °C
Enthalpy of Vaporization: 67.78 kJ/mol
Boiling Point: 446.7 °C at 760 mmHg
Vapour Pressure: 9.41E-08 mmHg at 25 °C
Melting point: 105-107 °C
Structure of 4-Chloro-6,7-bis(2-methoxyethoxy)quinazoline (CAS NO.183322-18-1):
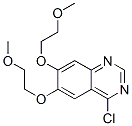
Product Category of 4-Chloro-6,7-bis(2-methoxyethoxy)quinazoline (CAS NO.183322-18-1):
Product Categor: Intermidiate of Erlotinib hydrochloride;API intermediates
4-Chloro-6,7-bis(2-methoxyethoxy)quinazoline Uses
4-Chloro-6,7-bis(2-methoxyethoxy)quinazoline (CAS NO.183322-18-1) is used as a chiral pharmaceutical intermediate.
4-Chloro-6,7-bis(2-methoxyethoxy)quinazoline Specification
4-Chloro-6,7-bis(2-methoxyethoxy)quinazoline , its cas register number is 183322-18-1. It also can be called 6,7-Bis(2-methoxyethoxy)-4-chloroquinazoline ; and 4-Chloro-6,7-(2-methoxyethoxy)quinazoline .
Related Products
- 4-Chloro-6-(1H-imidazol-1-yl)pyrimidine
- 4-Chloro-6-(2-hydroxyethylpiperazino)-2-methylamino-5-methylthiopyrimidine
- 4-Chloro-6-(2-methyl-1H-imidazol-1-yl)pyrimidine
- 4-Chloro-6-(dimethylamino)pyrimidine
- 4-Chloro-6-(methoxymethyl)-2-methyl-pyrimidine
- 4-Chloro-6-(phenylmethoxy)-2-pyrimidinamine
- 4-Chloro-6-(trifluoromethyl)pyridin-3-amine
- 4-Chloro-6-(trifluoromethyl)quinazoline
- 4-Chloro-6-(trifluoromethyl)quinoline
- 4-Chloro-6,7,8-trimethoxyquinazoline
- 183322-21-6
- 183322-47-6
- 1833-31-4
- 1833-51-8
- 1833-53-0
- 18335-33-6
- 18336-39-5
- 18336-75-9
- 18337-63-8
- 18337-64-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View