-
Name
4-Chlorobenzylamine
- EINECS 203-245-3
- CAS No. 104-86-9
- Article Data136
- CAS DataBase
- Density 1.16 g/cm3
- Solubility Not miscible in water.
- Melting Point 277-278 °C(Solv: N,N-dimethylformamide (68-12-2))
- Formula C7H8ClN
- Boiling Point 216.6 °C at 760 mmHg
- Molecular Weight 141.6
- Flash Point 92.6 °C
- Transport Information UN 2735
- Appearance clear slightly yellow liquid
- Safety 26-45-36/37/39
- Risk Codes 36/37/38-34
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, C
C
- Synonyms Benzylamine, p-chloro- (8CI);Benzenemethanamine, 4-chloro-;(4-chlorophenyl)methanamine;(4-chlorophenyl)methylazanium;Benzylamine, p-chloro-;p-Chlorobenzylamine;p-Chlorobenzyl amine;
- PSA 26.02000
- LogP 2.49900
Synthetic route

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; hydrogen In ethanol at 130℃; under 7500.75 Torr; for 12h; Autoclave; | 100% |
| With ammonia; hydrogen In methanol at 89.84℃; under 30003 Torr; for 3h; | 98% |
| With [pentamethylcyclopentadienyl*Ir(N-phenyl-2-pyridinecarboxamidate)Cl]; ammonium formate In methanol at 37℃; for 15h; chemoselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| With [Ru(H)(BH4)(CO)(PPh3)(3-(di-tert-butylphosphino)-N-((1-methyl-1H-imidazol-2 yl)methyl)propylamine)]; hydrogen In isopropyl alcohol at 70℃; for 3h; Inert atmosphere; Autoclave; | 99% |
| With ammonia; hydrogen In water; isopropyl alcohol at 80℃; under 15001.5 Torr; for 24h; Autoclave; | 98% |
| With C19H34Cl2CoN2P; hydrogen; sodium ethanolate; sodium triethylborohydride In benzene at 135℃; under 22502.3 Torr; for 36h; Autoclave; | 93% |

| Conditions | Yield |
|---|---|
| With (pyridine)(tetrahydroborato)zinc In tetrahydrofuran for 2.8h; Heating; | 96% |
| With sodium hydrogensulfate monohydrate; molybdenum(V) chloride; sodium cyanoborohydride In ethanol for 2.2h; Reflux; | 96% |
| With sodium tetrahydroborate at 20℃; for 0.0333333h; neat (no solvent, solid phase); | 95% |
-
-
27032-10-6
4-chlorobenzyl azide

-
-
104-86-9
4-chlorobenzylamine

| Conditions | Yield |
|---|---|
| With Amberlite IRA-400; borohydride form; copper(II) sulfate In methanol at 20℃; for 6h; Reduction; | 94% |
| With ammonium chloride; zinc In ethanol; water | |
| With hydrogen; palladium In tetrahydrofuran; N,N-dimethyl-formamide at 50℃; under 6080.41 Torr; for 22h; Inert atmosphere; | |
| With formic acid; 2,6-dimethyl-pyridine-3,5-dicarboxylic acid diethyl ester; N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 40h; Irradiation; | 84 %Chromat. |
-
-
623-03-0
4-Cyanochlorobenzene

-
A
-
21913-13-3
N,N-bis(4-chlorobenzyl)amine

-
B
-
104-86-9
4-chlorobenzylamine

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; nickel dichloride In ethanol at 20℃; for 0.0833333h; | A 7% B 85% |
| With methanol; cobalt(II) chloride; diborane at -10℃; for 0.5h; | A 9.4% B 71.1% |
| With methanol; cobalt(II) chloride; diborane for 0.5h; Ambient temperature; | A 52% B 27% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; potassium dihydrogenphosphate In tert-butyl methyl ether at 20℃; for 0.5h; Reagent/catalyst; Solvent; Autoclave; | 85% |
| With hexamethylenetetramine | |
| With hydrogenchloride; potassium hydride; 1,1,3,3-tetramethyldisilazane 1.) THF, 1 h, 0 deg C; 1 h, r.t.; Yield given. Multistep reaction; | |
| With ammonia at 25℃; Kinetics; Activation energy; Temperature; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; samarium diiodide In tetrahydrofuran for 0.0666667h; Ambient temperature; | A 6% B 77% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; C19H37IrN4(2+)*2I(1-) at 170℃; for 100h; Sealed tube; | 77% |
| With ammonia In toluene at 110℃; under 5250.53 Torr; for 20h; | 72% |
| Multi-step reaction with 2 steps 1: acetic acid; hydrogen bromide / 0.5 h / 0 °C 2: ammonia / ethanol; water / 4 h / 20 °C View Scheme |
-
-
131523-32-5
2-(4-chloro-benzyl)-1H-isoindole-1,3(2H)-dione

-
-
104-86-9
4-chlorobenzylamine

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In 1,4-dioxane at 110℃; Gabriel Amine Synthesis; Inert atmosphere; | 74% |
| With hydrazine hydrate In ethanol for 3h; Inert atmosphere; Reflux; | 42% |
| With hydrazine hydrate In methanol for 1h; Heating; |

| Conditions | Yield |
|---|---|
| With titanium tetrachloride; magnesium In tetrahydrofuran at 0 - 20℃; Inert atmosphere; | 72% |
| With sulfuric acid at 35 - 40℃; Electrolysis.an einer Bleikathode; | |
| With 1,10-Phenanthroline; diethoxymethylane; iron(II) acetate In toluene at 100℃; for 28h; Inert atmosphere; chemoselective reaction; | 60 %Chromat. |
-
-
67-56-1
methanol

-
-
27032-10-6
4-chlorobenzyl azide

-
A
-
15184-98-2
N,N-dimethyl-4-chlorobenzylamine

-
B
-
104-86-9
4-chlorobenzylamine

-
C
-
104-11-0
N-methyl-4-chlorobenzylamine

| Conditions | Yield |
|---|---|
| With trans-RuCl(phenpyra-Me)(PPh3)2PF6; sodium hydroxide at 125℃; for 2.5h; Sealed tube; Inert atmosphere; Glovebox; | A 5 %Chromat. B 7 %Chromat. C 69% |


| Conditions | Yield |
|---|---|
| With potassium hydroxide; samarium diiodide In tetrahydrofuran for 0.05h; Ambient temperature; | A 12% B 56% |

| Conditions | Yield |
|---|---|
| With D-glucose; E. coli LZ220; ammonia; oxygen; ammonium chloride In aq. phosphate buffer at 30℃; for 24h; pH=8; Green chemistry; Enzymatic reaction; | 47% |

-
A
-
33537-99-4
6‐chloro‐1,2,3,4‐tetrahydroisoquinoline

-
B
-
104-86-9
4-chlorobenzylamine

| Conditions | Yield |
|---|---|
| With aluminium trichloride In decalin at 180℃; for 2h; | A 45% B n/a |
-
-
623-03-0
4-Cyanochlorobenzene

-
A
-
21913-13-3
N,N-bis(4-chlorobenzyl)amine

-
B
-
104-86-9
4-chlorobenzylamine

-
C
-
873-76-7
para-Chlorobenzyl alcohol

| Conditions | Yield |
|---|---|
| With nickel dichloride; zinc In methanol for 4h; Heating; | A 5% B 37.4% C 39.2% |
| With nickel dichloride; zinc In methanol for 4h; Heating; | A 5% B 37.4% C 39.2% |

-
-
41097-37-4
(1E, 2E)-1,2-bis (4-chlorobenzylidene) hydrazine

-
A
-
86212-34-2, 91044-20-1, 98674-96-5, 74641-30-8
(1R,2S)-1,2-bis(4-chlorophenyl)ethane-1,2-diamine

-
B
-
104-86-9
4-chlorobenzylamine

| Conditions | Yield |
|---|---|
| With methanesulfonic acid; zinc In tetrahydrofuran at 25℃; for 8h; reduction; | A n/a B 35% C n/a |
| With titanium tetrachloride; zinc In tetrahydrofuran at 25℃; for 8h; reduction; | A n/a B 13% C n/a |

-
-
3848-36-0
4-chlorobenzaldoxime

-
A
-
86212-34-2, 91044-20-1, 98674-96-5, 74641-30-8
(1R,2S)-1,2-bis(4-chlorophenyl)ethane-1,2-diamine

-
B
-
104-86-9
4-chlorobenzylamine

| Conditions | Yield |
|---|---|
| With methanesulfonic acid; zinc In acetonitrile at 25℃; reduction; | A n/a B 32% C n/a |
| With titanium tetrachloride; zinc In tetrahydrofuran at 25℃; reduction; | A n/a B 3% C n/a |

-
-
17332-59-1
N-(4-chloro-benzyl)-phthalamic acid

-
-
104-86-9
4-chlorobenzylamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
96634-13-8
(4-chloro-benzyl)-hexamethylenetetraminium; chloride

-
-
104-86-9
4-chlorobenzylamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; methanol |

| Conditions | Yield |
|---|---|
| anschliessenden Behandeln mit wss.HCl; |
-
-
33499-37-5, 54615-09-7, 87861-05-0
4-chlorobenzaldehyde-O-methyl oxime

-
-
104-86-9
4-chlorobenzylamine

| Conditions | Yield |
|---|---|
| (i) B2H6, THF, (ii) aq. KOH; Multistep reaction; |
-
-
6342-46-7
(E)-N′-(4′-chlorobenzylidene)isonicotinohydrazide

-
A
-
586-95-8
pyridine-4-methanol

-
B
-
104-86-9
4-chlorobenzylamine

| Conditions | Yield |
|---|---|
| In water; N,N-dimethyl-formamide Mechanism; polarographic reduction,; |

| Conditions | Yield |
|---|---|
| With tetramethylammonium bromide Mechanism; polarographic reduction at pH=3, other supporting electrolytes; values of diffusion current constant; |

| Conditions | Yield |
|---|---|
| With tetramethylammonium bromide Mechanism; polarographic reduction at pH=3, other supporting electrolytes; values of diffusion current constant; |

| Conditions | Yield |
|---|---|
| With sulfuric acid In water at 60 - 92℃; Kinetics; Mechanism; Thermodynamic data; ΔH(excit.), ΔS(excit.); |


-
-
114444-46-1
C14H13NO

-
A
-
124225-44-1
1-methyl-4-(phenylacetyl)pyridinium cation

-
B
-
104-86-9
4-chlorobenzylamine

| Conditions | Yield |
|---|---|
| In water at 25℃; Equilibrium constant; Rate constant; |

-
-
102677-74-7
3-amino-4-chlorobenzylamine dihydrochloride

-
-
104-86-9
4-chlorobenzylamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hypophosphorous acid; sodium nitrite 2.) 15 min; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With iodide; hydrogen cation In water at 25℃; Rate constant; pH dependence (pH = 0.5-3.0), μ = 1.0 with KCl; |
-
-
97732-00-8
N-(4-chlorobenzyl)-S,S-dimethylsulfilimmonium chloride

-
A
-
75-18-3
dimethylsulfide

-
B
-
104-86-9
4-chlorobenzylamine

| Conditions | Yield |
|---|---|
| With 3-nitro-5-thiobenzoic acid; hydrogen cation In water at 25℃; Rate constant; var. reductant thiol anions, ionic strength: μ = 1.0 with KCl, catalytic constants for buffer, leaving group effects; |
-
-
104-86-9
4-chlorobenzylamine

-
-
98077-17-9
4-Ethoxy-2,3,5,6,7,8-hexahydro-chinazolin-2-on

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 20.5h; Heating; | 100% |
-
-
104-86-9
4-chlorobenzylamine

-
-
31264-06-9
N-(4-chlorobenzylidene)-4-chlorobenzylamine

| Conditions | Yield |
|---|---|
| With oxygen; methylene blue; potassium carbonate In acetonitrile at 20℃; for 10h; Irradiation; Green chemistry; | 100% |
| With oxygen at 100℃; for 8h; Time; Schlenk technique; | 99% |
| With oxygen; 2Co(2+)*2C12H6O4(2-)*C34H12Cl4N4O4*4C3H7NO In N,N-dimethyl-formamide at 40℃; for 6h; Irradiation; | 99% |

-
-
104-86-9
4-chlorobenzylamine

-
-
694479-04-4
N-(4-chlorobenzyl)-1-(3-methylphenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide

| Conditions | Yield |
|---|---|
| With 1-hydroxy-7-aza-benzotriazole; 2-(3H-[1,2,3]triazolo[4,5-b]pyridin-3-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate(V); diisopropylamine In dichloromethane at 20℃; for 1h; | 100% |
-
-
104-86-9
4-chlorobenzylamine

-
-
305324-43-0
N-[(tert-butoxy)carbonyl]-N-(2-oxo-3-phenylpropyl)glycine

-
-
305324-50-9
4-[(tert-butoxy)carbonyl]-1-[(4-chlorophenyl)methyl]-6-(phenylmethyl)piperazin-2-one

| Conditions | Yield |
|---|---|
| Stage #1: 4-chlorobenzylamine; N-[(tert-butoxy)carbonyl]-N-(2-oxo-3-phenylpropyl)glycine With sodium tris(acetoxy)borohydride; acetic acid at 25℃; Condensation; Stage #2: With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; Cyclization; | 100% |
-
-
104-86-9
4-chlorobenzylamine

-
-
305324-44-1
N-[(tert-butoxy)carbonyl]-N-(2-oxo-4-phenylbutyl)glycine

-
-
305324-55-4
4-[(tert-butoxy)carbonyl]-1-[(4-chlorophenyl)methyl]-6-(2-phenylethyl)piperazin-2-one

| Conditions | Yield |
|---|---|
| Stage #1: 4-chlorobenzylamine; N-[(tert-butoxy)carbonyl]-N-(2-oxo-4-phenylbutyl)glycine With sodium tris(acetoxy)borohydride; acetic acid at 25℃; Condensation; Stage #2: With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 25℃; Cyclization; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-chlorobenzylamine; 1,1'-Thiocarbonyldiimidazole In acetonitrile at 20℃; for 1h; Stage #2: methyl iodide In acetonitrile at 20℃; | 100% |

-
-
931-53-3
Cyclohexyl isocyanide

-
-
123-38-6
propionaldehyde

-
-
104-86-9
4-chlorobenzylamine

-
-
88-75-5
2-hydroxynitrobenzene

| Conditions | Yield |
|---|---|
| In methanol microwave irradiation; | 100% |
| In toluene at 60℃; for 20h; Ugi-Smiles coupling; | 90% |
| In methanol at 40℃; for 20h; Ugi reaction; | 74% |


| Conditions | Yield |
|---|---|
| In toluene | 100% |
| In toluene at 20℃; for 2.25h; Heating / reflux; | 100% |
| In toluene at 20℃; for 2.25h; Heating / reflux; | 100% |
| With triethylamine In dichloromethane; toluene at -20 - 25℃; for 4.08333h; Inert atmosphere; |
-
-
626-05-1
2,6-Dibromopyridine

-
-
104-86-9
4-chlorobenzylamine

-
-
866546-12-5
(6-bromopyridin-2-yl)(4-chlorobenzyl)amine

| Conditions | Yield |
|---|---|
| at 150℃; for 0.5h; Neat (no solvent); Microwave irradiation; | 100% |
-
-
104-86-9
4-chlorobenzylamine

-
-
84358-13-4
N-[(tert-butoxy)carbonyl]piperidine-4-carboxylic acid

-
-
881833-22-3
tert-butyl 4-(4-chlorobenzylcarbamoyl)piperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With 1-hydroxy-7-aza-benzotriazole; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In tetrahydrofuran at 20℃; for 16h; | 100% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 15h; | 88% |
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In dichloromethane at 20℃; for 18h; | 72% |
-
-
6342-56-9
Pyruvic aldehyde dimethyl acetal

-
-
104-86-9
4-chlorobenzylamine

-
-
919994-55-1
(4-chloro-benzyl)-[2,2-dimethoxy-1-methyl-eth-(Z)-ylidene]-amine

| Conditions | Yield |
|---|---|
| In toluene for 3h; Heating / reflux; | 100% |
-
-
79099-07-3
N-tert-butyloxycarbonylpiperidin-4-one

-
-
104-86-9
4-chlorobenzylamine

-
-
849106-37-2
4-(4-chloro-benzylamino)-piperidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride; acetic acid In 1,2-dichloro-ethane | 100% |
| With sodium tris(acetoxy)borohydride; acetic acid In 1,2-dichloro-ethane at 20℃; for 2h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| In water at 20℃; for 2h; Michael-type addition; | 100% |
| In dichloromethane at 20℃; for 0.166667h; | |
| In methanol for 0.5h; Reflux; | |
| In dichloromethane at 20℃; Inert atmosphere; |
-
-
104-86-9
4-chlorobenzylamine

-
-
532-55-8
Benzoyl isothiocyanate

-
-
145383-00-2
N-((4-chlorobenzyl)carbamothioyl)benzamide

| Conditions | Yield |
|---|---|
| 100% | |
| In diethyl ether at 0 - 20℃; for 2h; | |
| In acetone at 20℃; |
-
-
100032-83-5
(Z)-3-ethoxy-3-[(2-nitrophenyl)hydrazono]propanoic acid ethyl ester

-
-
104-86-9
4-chlorobenzylamine

| Conditions | Yield |
|---|---|
| at 50℃; for 1h; Inert atmosphere; Microwave irradiation; | 100% |
-
-
27631-29-4
2-chloro-6,7-dimethoxy-3H-quinazolin-4-one

-
-
104-86-9
4-chlorobenzylamine

-
-
864291-36-1
2-chloro-N-(4-chlorobenzyl)-6,7-dimethoxyquinazolin-4-amine

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloro-6,7-dimethoxy-3H-quinazolin-4-one; 4-chlorobenzylamine In N,N-dimethyl-formamide at 20℃; for 29h; Stage #2: With sodium hydroxide In water | 100% |
-
-
329-59-9
4-fluoro-3-nitro-benzoic acid methyl ester

-
-
104-86-9
4-chlorobenzylamine

-
-
174422-22-1
methyl 4-(4-chlorobenzylamine)-3-nitrobenzoic acid ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; for 5h; | 100% |
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; for 5h; | 100% |
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran for 17h; Reflux; | |
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 50℃; for 16h; | |
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 150℃; for 0.0833333h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| Stage #1: carbon disulfide; 4-chlorobenzylamine In diethyl ether for 0.25h; Cooling with ice; Stage #2: With dicyclohexyl-carbodiimide In diethyl ether at 20℃; for 24h; | 100% |
| Stage #1: carbon disulfide; 4-chlorobenzylamine With triethylamine In tetrahydrofuran at 0 - 20℃; for 1.75h; Stage #2: With p-toluenesulfonyl chloride In tetrahydrofuran at 0 - 20℃; for 1h; | 85% |
| With dmap; di-tert-butyl dicarbonate; triethylamine In ethanol at 0 - 20℃; for 1h; | 71% |

| Conditions | Yield |
|---|---|
| Stage #1: Veratric acid With 1-hydroxy-pyrrolidine-2,5-dione In dichloromethane at -10 - 0℃; for 0.166667h; Stage #2: With dicyclohexyl-carbodiimide In dichloromethane for 2h; Reflux; Stage #3: 4-chlorobenzylamine With triethylamine In methanol; dichloromethane for 2h; Reflux; | 99.5% |

| Conditions | Yield |
|---|---|
| With oxygen In acetone at 20℃; for 3.2h; Electrochemical reaction; | 99% |
| With potassium hydroxide; nickel copper formate; (Bu4N)2S2O8 In 1,2-dichloro-ethane at 20℃; for 13h; Oxidation; | 95% |
| With [hydroxy(tosyloxy)iodo]benzene; ammonium acetate In water; acetonitrile at 80℃; for 3h; | 92% |
-
-
104-88-1
4-chlorobenzaldehyde

-
-
104-86-9
4-chlorobenzylamine

-
-
31264-06-9
N-(4-chlorobenzylidene)-4-chlorobenzylamine

| Conditions | Yield |
|---|---|
| In dichloromethane at 40℃; under 760.051 Torr; for 1.5h; Molecular sieve; | 99% |
| With magnesium sulfate In dichloromethane for 3h; Reflux; | 95% |
| In toluene for 24h; Condensation; Heating; | 56% |
-
-
104-86-9
4-chlorobenzylamine

-
-
54856-20-1
N-[(2-methoxycarbonylbenzene)sulfenyl]-1,2-benzisothiazolin-3-one

-
-
4322-81-0
2-(4-chlorobenzyl)benzo[d]isothiazol-2(3H)-one

| Conditions | Yield |
|---|---|
| In toluene at 100℃; for 3h; | 99% |
-
-
1201482-15-6
(R)-3-((S)-4-benzyl-2-oxooxazolidine-3-carbonyl)hex-5-enoic acid

-
-
104-86-9
4-chlorobenzylamine

-
-
1201482-18-9
(R)-3-((S)-4-benzyl-2-oxooxazolidine-3-carbonyl)-N-(4-chlorobenzyl)hex-5-enamide

| Conditions | Yield |
|---|---|
| Stage #1: (R)-3-((S)-4-benzyl-2-oxooxazolidine-3-carbonyl)hex-5-enoic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; for 0.5h; Stage #2: 4-chlorobenzylamine With dmap In dichloromethane | 99% |
-
-
82979-45-1
methyl 2-chloro-2-cyclopropylideneacetate

-
-
104-86-9
4-chlorobenzylamine

-
-
1239987-62-2
methyl 2-(4-chlorobenzylamino)cyclobut-1-enecarboxylate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; lithium iodide In N,N-dimethyl-formamide at 0 - 20℃; for 72h; Michael condensation; Inert atmosphere; | 99% |
-
-
104-86-9
4-chlorobenzylamine

-
-
372-31-6
ethyl 4,4,4-trifluoroacetoacetate

-
-
1384849-55-1
ethyl 3-(4-chlorobenzyl)amino-4,4,4-trifluorobut-2-enoate

| Conditions | Yield |
|---|---|
| Stage #1: 4-chlorobenzylamine With acetic acid In chloroform at 20℃; for 0.0833333h; Stage #2: ethyl 4,4,4-trifluoroacetoacetate In chloroform for 5h; Reflux; | 99% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 30℃; for 3h; Green chemistry; | 99% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 25℃; for 12h; | 99% |

| Conditions | Yield |
|---|---|
| With phenylsilane; C14H24N4Si*HI In benzene-d6 at 40℃; Inert atmosphere; Schlenk technique; | 99% |
| With Au-TiO2; hydrogen In N,N-dimethyl acetamide at 120℃; for 5h; | 94% |
| With tris(2-diphenylphosphinoethyl)phosphine; hydrogen; potassium carbonate; cobalt(II) perchlorate hexahydrate In ethanol at 140℃; under 45004.5 Torr; for 24h; Autoclave; Green chemistry; | 90% |
4-Chlorobenzylamine Specification
The IUPAC name of this chemical is 4-Chlorobenzylamine. With the CAS registry number 104-86-9 and EINECS registry number 203-245-3, it is also named as Benzenemethanamine, 4-chloro-. In addition, the molecular formula is C7H8ClN and the molecular weight is 141.60. It is a kind of clear slightly yellow liquid and belongs to the classes of Anilines, Aromatic Amines and Nitro Compounds; C7; Nitrogen Compounds. What's more, it should be stored in sealed container, and put in a cool and dry place. The storage place must stay away from oxidant, the fire, water source and heat source.
Physical properties about this chemical are: (1)ACD/LogP: 1.68; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -1.31; (4)ACD/LogD (pH 7.4): -0.04; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 3.67; (9)#H bond acceptors: 1; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 3.24 Å2; (13)Index of Refraction: 1.566; (14)Molar Refractivity: 39.59 cm3; (15)Molar Volume: 121.3 cm3; (16)Polarizability: 15.69×10-24cm3; (17)Surface Tension: 42 dyne/cm; (18)Density: 1.166 g/cm3; (19)Flash Point: 92.6 °C; (20)Enthalpy of Vaporization: 45.3 kJ/mol; (21)Boiling Point: 216.6 °C at 760 mmHg; (22)Vapour Pressure: 0.139 mmHg at 25 °C.
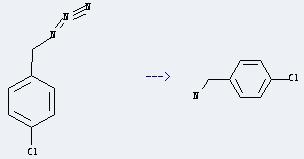
Preparation of 4-Chlorobenzylamine: it can be prepared by p-Chlorobenzyl azide. This reaction will need reagent Amberlite IRA-400, borohydride form and CuSO4*5H2O and solvent methanol. The reaction time is 6 hours at reaction temperature of 20 °C. The yield is about 94 %.
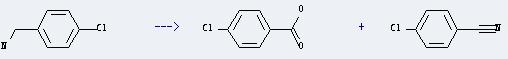
Uses of 4-Chlorobenzylamine: it can be used to get 4-Chloro-benzoic acid and 4-Chloro-benzonitrile. This reaction will need reagents tert-butylhydroperoxide and silica-supported selenamide, and solvent 2-methyl-propan-2-ol. The reaction time is 20 hours by heating. The yield is about 87 %.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin and it can cause burns. During using it, you should wear suitable protective clothing, gloves and eye/face protection. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice. And in case of accident or if you feel unwell, seek medical advice immediately.
You can still convert the following datas into molecular structure:
(1)SMILES: Clc1ccc(cc1)CN
(2)InChI: InChI=1/C7H8ClN/c8-7-3-1-6(5-9)2-4-7/h1-4H,5,9H2
(3)InChIKey: YMVFJGSXZNNUDW-UHFFFAOYAN
Related Products
- 4-Chlorobenzylamine
- 104-87-0
- 10487-10-2
- 10487-11-3
- 104872-06-2
- 104874-50-2
- 10487-71-5
- 104-88-1
- 104881-72-3
- 104883-49-0
- 10488-68-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View