-
Name
4-Nitrosalicylic acid
- EINECS 210-584-0
- CAS No. 619-19-2
- Article Data41
- CAS DataBase
- Density 1.632 g/cm3
- Solubility
- Melting Point 235-239 °C(lit.)
- Formula C7H5NO5
- Boiling Point 389.046 °C at 760 mmHg
- Molecular Weight 183.12
- Flash Point 179.176 °C
- Transport Information
- Appearance White to yellow crystalline powder
- Safety 26-36
- Risk Codes 22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Salicylicacid, 4-nitro- (7CI,8CI);2-Hydroxy-4-nitrobenzoic acid;4-Nitro-2-hydroxybenzoic acid;NSC 882;p-Nitrosalicylicacid;
- PSA 103.35000
- LogP 1.52180
Synthetic route

| Conditions | Yield |
|---|---|
| With boron tribromide In dichloromethane | 97% |
| With sulfuric acid | |
| With hydrogen bromide | |
| With hydrogen bromide; acetic acid at 90℃; for 72h; |

| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 20℃; for 2h; | 97% |

| Conditions | Yield |
|---|---|
| With pyridine; water; potassium carbonate; copper for 0.25h; sonication; | 96% |
| With pyridine; copper; potassium carbonate In water for 2h; Heating; | 90% |
| With quicklime; water; copper at 160 - 170℃; | |
| With copper(l) iodide; copper; potassium carbonate | |
| With calcium hydroxide; copper diacetate In water at 160℃; for 7h; |
-
-
57356-40-8
2-(hydroxymethyl)-5-nitrophenol

-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With sodium hypochlorite; water at 25℃; for 0.5h; | 96% |
| With oxygen In acetonitrile at 20℃; for 8.5h; Catalytic behavior; | 85% |

| Conditions | Yield |
|---|---|
| With oxygen; potassium acetate; palladium diacetate; p-benzoquinone In N,N-dimethyl acetamide at 115℃; under 3800.26 Torr; for 15h; | 91% |
| With oxygen; potassium acetate; p-benzoquinone; palladium diacetate In ISOPROPYLAMIDE at 115℃; under 3800.26 Torr; for 15h; Product distribution / selectivity; | 91% |
| With [Fe(II)(N,N'-dimethyl-N,N'-bis(2-pyridylmethyl)-1,2-ethylenediamine)(CH3CN)2](ClO4)2; dihydrogen peroxide In water; acetonitrile at 20℃; Inert atmosphere; regioselective reaction; | 68 %Spectr. |

| Conditions | Yield |
|---|---|
| With sodium perborate hexahydrate; hydroquinone; sodium nitrite In neat (no solvent) for 0.0333333h; Molecular sieve; Microwave irradiation; regioselective reaction; | 75% |
| With potassium hydrogensulfate; potassium metaperiodate; silica gel; sodium nitrite In neat (no solvent) for 0.0833333h; Reagent/catalyst; Solvent; Microwave irradiation; | 69% |
| With potassium hydrogensulfate; potassium metaperiodate; sodium nitrite In water; acetonitrile at 44.84℃; Kinetics; Temperature; |
-
-
13684-28-1
2-hydroxy-4-nitro-benzoic acid methyl ester

-
-
62-23-7
4-nitro-benzoic acid

-
A
-
619-50-1
4-nitrobenzoic acid methyl ester

-
B
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4-nitro-benzoic acid With potassium carbonate In N,N-dimethyl acetamide at 90℃; for 0.5h; Stage #2: 2-hydroxy-4-nitro-benzoic acid methyl ester at 90℃; for 24h; | A 32% B n/a |


| Conditions | Yield |
|---|---|
| With potassium permanganate |
-
-
50603-43-5
7-nitro-4H-benzo[1,3]dioxine

-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With potassium permanganate; acetic acid |
-
-
39835-14-8
2-hydroxy-4-nitro-benzonitrile

-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid Siedetemperatur; | |
| With hydrogenchloride at 160℃; Reinigung ueber das Barium-Salz; | |
| With sulfuric acid; acetic acid Siedetemperatur; |
-
-
16426-64-5
2-bromo-4-nitrobenzoic acid

-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With barium dihydroxide; sodium hydroxide; copper (I) acetate |
-
-
101084-96-2
2-methoxy-4-nitrobenzonitrile

-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid | |
| With sulfuric acid; hydrogen bromide; acetic acid |

-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate |

| Conditions | Yield |
|---|---|
| Diazotization; | |
| With sulfuric acid; sodium nitrite Diazotization; |
-
-
129973-05-3
2-(2-Hydroxy-4-nitro-benzoylamino)-2-methyl-propionic acid

-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid 1) reflux, 2) 3 deg C; Yield given; |

| Conditions | Yield |
|---|---|
| With Tris-HCl buffer; water; calcium(II) ion; sodium chloride; Agkistrodon piscivorus piscivorus In acetonitrile at 37℃; Rate constant; pH = 8.0; |
-
-
7647-01-0
hydrogenchloride

-
-
5453-86-1
methyl 6-nitro-1,2-benzisoxazole-3-carboxylate

-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| at 150℃; |

| Conditions | Yield |
|---|---|
| With potassium carbonate at 210℃; under 29420.3 Torr; |

-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; acetic acid |

-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 150℃; |

-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With potassium permanganate |

| Conditions | Yield |
|---|---|
| at 160 - 170℃; |


-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With sodium-; copper diacetate; magnesium oxide at 170℃; weiteres Reagens: Wasser; |
-
-
7647-01-0
hydrogenchloride

-
-
98550-12-0
2-hydroxy-4-nitro-benzohydroxamic acid

-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| at 150℃; |

| Conditions | Yield |
|---|---|
| With Streptomyces thioluteus para-aminobenzoate N-oxygenase |
-
-
52944-16-8
2-(4-nitrobenzoylamino)isobutyric acid

-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1) Cu(0)/O2/trimethylamine N-oxide, 2) 0.5N HCl / 1) CH3CN, 75 deg C, 1.8h 2: 15percent aq. H2SO4 / 1) reflux, 2) 3 deg C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1) NaOH, 2) 5N HCl / 1) H2O/THF, a) 1 deg C, 2.5h, b) 20 deg C, 1h, 2) 0 deg C 2: 1) Cu(0)/O2/trimethylamine N-oxide, 2) 0.5N HCl / 1) CH3CN, 75 deg C, 1.8h 3: 15percent aq. H2SO4 / 1) reflux, 2) 3 deg C View Scheme |
-
-
192181-49-0
4-nitro-2-phenoxybenzoic acid

-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: HNO3 2: aqueous K2CO3 View Scheme |
-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

-
-
39614-82-9
2-hydroxy-4-nitrobenzoyl chloride

| Conditions | Yield |
|---|---|
| With thionyl chloride at 90℃; for 1h; | 100% |
| With thionyl chloride In chloroform at 0℃; for 5h; Heating / reflux; | 75% |
| With thionyl chloride; benzene |
-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

-
-
18107-18-1
diazomethyl-trimethyl-silane

-
-
13684-28-1
2-hydroxy-4-nitro-benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| In methanol; hexane; toluene at 20℃; for 1.25h; | 100% |
| In methanol; hexane; toluene at 20℃; for 1.25h; | 100% |

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 20℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 70℃; for 12h; | 100% |
-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

-
-
77-78-1
dimethyl sulfate

-
-
39106-79-1
methyl 2-methoxy-4-nitrobenzoate

| Conditions | Yield |
|---|---|
| With potassium carbonate for 4h; Reflux; | 99% |
| With tetrabutylammomium bromide In dichloromethane for 10h; Ambient temperature; Yield given; |
-
-
67-56-1
methanol

-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

-
-
13684-28-1
2-hydroxy-4-nitro-benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| With sulfuric acid at 65℃; for 96h; Inert atmosphere; | 98% |
| With sulfuric acid for 12h; pH=<= 1; Reflux; | 98% |
| With sulfuric acid at 70℃; | 96% |
-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

-
-
57356-40-8
2-(hydroxymethyl)-5-nitrophenol

| Conditions | Yield |
|---|---|
| With borane-THF In tetrahydrofuran at 0 - 23℃; for 18h; | 98% |
| With borane-THF | 98% |
| With dimethylsulfide borane complex In tetrahydrofuran at 20℃; for 16h; Cooling with ice; | 97.5% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-hydroxy-4-nitrobenzoic acid With thionyl chloride In methanol Stage #2: With hydrogen; palladium 10% on activated carbon In ethyl acetate | 98% |
| Multi-step reaction with 2 steps 1: 95 percent / SOCl2 2: 98 percent / H2 / Pd/C / ethyl acetate View Scheme | |
| Multi-step reaction with 2 steps 1: nickel-aluminium-alloy; aq. NaOH solution 2: sulfuric acid View Scheme |
-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

-
-
75-03-6
ethyl iodide

-
-
910572-96-2
2-ethoxy-4-nitro-benzoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In butanone at 80℃; for 16h; | 97% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; |
-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

-
-
13684-28-1
2-hydroxy-4-nitro-benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| With thionyl chloride In methanol at 0℃; for 6h; Reflux; | 97% |
| With sulfuric acid In methanol |

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide | 97% |

| Conditions | Yield |
|---|---|
| With aminomethyl polystyrene resin formic acid salt; zinc In methanol at 20℃; for 2h; | 96% |
| With ammoniummethyl polystyrene resin formate; palladium on activated charcoal In methanol at 20℃; for 5h; | 95% |
| With polymer-supported formate; magnesium In methanol at 20℃; for 3h; | 94% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

-
-
39106-79-1
methyl 2-methoxy-4-nitrobenzoate

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; for 4h; Methylation; | 96% |
-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

-
-
106-95-6
allyl bromide

-
-
735351-47-0
2-allyloxy-4-nitrobenzoic acid allyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 12h; Inert atmosphere; | 90% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 22h; | 90% |
| Stage #1: 2-hydroxy-4-nitrobenzoic acid With sodium hydride In N,N-dimethyl-formamide at 20℃; for 0.333333h; Stage #2: allyl bromide In N,N-dimethyl-formamide at 20℃; for 16h; | 76% |
| Stage #1: 2-hydroxy-4-nitrobenzoic acid With sodium hydride In DMF (N,N-dimethyl-formamide) at 20℃; for 0.333333h; Stage #2: allyl bromide In DMF (N,N-dimethyl-formamide) at 20℃; for 16h; Stage #3: With hydrogenchloride In DMF (N,N-dimethyl-formamide); water |
-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

-
-
74-88-4
methyl iodide

-
-
13684-28-1
2-hydroxy-4-nitro-benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 2-hydroxy-4-nitrobenzoic acid With potassium hydrogencarbonate In N,N-dimethyl-formamide at 20℃; for 0.166667h; Inert atmosphere; Stage #2: methyl iodide In N,N-dimethyl-formamide at 20℃; for 6h; Time; | 90% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 20℃; for 1h; | 84% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 40℃; for 8h; | 90% |
-
-
64-17-5
ethanol

-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

-
-
78987-51-6
2-hydroxy-4-nitro-benzoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With thionyl chloride at 0 - 20℃; Product distribution / selectivity; Heating / reflux; | 89% |
| With thionyl chloride Reflux; |

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide | 88% |
| Multi-step reaction with 2 steps 1: B2H6 / tetrahydrofuran 2: MnO2 / CHCl3 / Heating View Scheme | |
| Multi-step reaction with 4 steps 1: potassium carbonate / 4 h / Reflux 2: diisobutylaluminium hydride / tetrahydrofuran / 1 h / 0 °C 3: dipyridinium dichromate / dichloromethane / 3 h / 40 °C 4: phosphorus tribromide / dichloromethane / 4 h / -78 - 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 16h; Inert atmosphere; | 88% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-hydroxy-4-nitrobenzoic acid With N-ethyl-N,N-diisopropylamine; HATU In dichloromethane at 0 - 20℃; for 1.5h; Stage #2: dimethyl amine In dichloromethane | 88% |
| Stage #1: 2-hydroxy-4-nitrobenzoic acid With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In dichloromethane at 20℃; for 1.5h; Stage #2: dimethyl amine In dichloromethane at 20℃; | 71% |
-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

-
-
17336-10-6
2-acetoxy-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With dmap; acetic anhydride; triethylamine In ethyl acetate at 80℃; | 87% |
-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

-
-
75-65-0
tert-butyl alcohol

-
-
1356600-78-6
tert-butyl 2-hydroxy-4-nitrobenzoate

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In tetrahydrofuran at 20℃; for 20h; Inert atmosphere; | 87% |
| With dmap; dicyclohexyl-carbodiimide In tetrahydrofuran Reflux; | 81.7% |
| With dmap; dicyclohexyl-carbodiimide In tetrahydrofuran at 60℃; | 73% |
| With benzotriazol-1-ol; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide; triethylamine In tert-butyl alcohol at 10 - 35℃; | 2.3 g |
-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

-
-
67-63-0
isopropyl alcohol

-
-
1319746-14-9
2-isopropoxy-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 100℃; for 2h; | 87% |
-
-
619-19-2
2-hydroxy-4-nitrobenzoic acid

-
-
100-39-0
benzyl bromide

-
-
5340-21-6
2-(benzyloxy)-4-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 16h; Inert atmosphere; | 83% |

| Conditions | Yield |
|---|---|
| With phosphorus trichloride Microwave irradiation; Reflux; | 83% |
4-Nitrosalicylic acid Consensus Reports
Reported in EPA TSCA Inventory.
4-Nitrosalicylic acid Specification
The 4-Nitrosalicylic acid, with the CAS registry number 619-19-2, is also known as 4-Nitro-2-hydroxybenzoic acid. It belongs to the product categories of Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts; C7; Carbonyl Compounds; Carboxylic Acids. Its EINECS number is 210-584-0. This chemical's molecular formula is C7H5NO5 and molecular weight is 183.12. What's more, its systematic name is 2-Hydroxy-4-nitrobenzoic acid.
Physical properties of 4-Nitrosalicylic acid are: (1)ACD/LogP: 2.859; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -0.27; (4)ACD/LogD (pH 7.4): -0.29; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 1.00; (8)ACD/KOC (pH 7.4): 1.00; (9)#H bond acceptors: 6; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 103.35 Å2; (13)Index of Refraction: 1.664; (14)Molar Refractivity: 41.61 cm3; (15)Molar Volume: 112.234 cm3; (16)Polarizability: 16.495×10-24cm3; (17)Surface Tension: 84.1 dyne/cm; (18)Density: 1.632 g/cm3; (19)Flash Point: 179.176 °C; (20)Enthalpy of Vaporization: 67.326 kJ/mol; (21)Boiling Point: 389.046 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
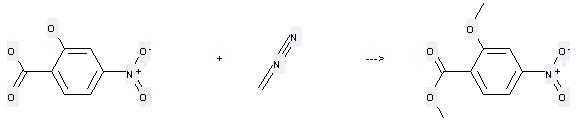
Uses of 4-Nitrosalicylic acid: it can be used to produce 2-methoxy-4-nitro-benzoic acid methyl ester at the temperature of 20 °C. It will need solvent diethyl ether with the reaction time of 4 hours. The yield is about 96%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. It is harmful if swallowed. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(O)c1ccc(cc1O)[N+]([O-])=O
(2)Std. InChI: InChI=1S/C7H5NO5/c9-6-3-4(8(12)13)1-2-5(6)7(10)11/h1-3,9H,(H,10,11)
(3)Std. InChIKey: UKWUOTZGXIZAJC-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 375mg/kg (375mg/kg) | Zeitschrift fuer Naturforschung, Teil B: Anorganische Chemie, Organische Chemie, Biochemie, Biophysik, Biologie. Vol. 6B, Pg. 183, 1951. | |
| mouse | LD50 | oral | 650mg/kg (650mg/kg) | Zeitschrift fuer Naturforschung, Teil B: Anorganische Chemie, Organische Chemie, Biochemie, Biophysik, Biologie. Vol. 6B, Pg. 183, 1951. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View