-
Name
D-2-Phenylglycine
- EINECS 212-876-3
- CAS No. 875-74-1
- Article Data90
- CAS DataBase
- Density 1.246 g/cm3
- Solubility 0.3 g/100mL in water
- Melting Point 302 °C (dec.)(lit.)
- Formula C8H9NO2
- Boiling Point 288.7 °C at 760 mmHg
- Molecular Weight 151.165
- Flash Point 128.4 °C
- Transport Information
- Appearance white crystalline powder
- Safety 22-24/25-45-36/37/39-26
- Risk Codes 23/24/25-36/37/38
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms Benzeneaceticacid, a-amino-, (R)-;(R)-a-Phenylglycine;D-(-)-2-Phenylglycine;D-(-)-Aminophenylacetic acid;D-(-)-Phenylglycine;D-(-)-a-Aminophenylacetic acid;D-(-)-a-Phenylglycine;D-2-Phenylglycine;D-C-Phenylglycine;D-Phenylglycine;D-a-Aminophenylacetic acid;Glycine,2-phenyl-, D- (8CI);(-)-(R)-Phenylglycine;(-)-Phenylglycine;(2R)-Amino-2-phenylethanoic acid;(R)-(-)-2-Phenylglycine;(R)-2-Amino-2-(phenyl)ethanoic acid;(R)-2-Amino-2-phenylacetic acid;(R)-2-Phenylglycine;(R)-Phenylglycine;
- PSA 63.32000
- LogP 1.47130
Synthetic route
-
-
29125-25-5
(R)-2-azido-2-phenylacetic acid

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In water; acetic acid under 760 Torr; for 3h; | 97% |

| Conditions | Yield |
|---|---|
| With nitrilase AY487533 In aq. acetate buffer at 20℃; for 1h; pH=8; Enzymatic reaction; enantioselective reaction; | 96.5% |

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In water at 110℃; for 3h; optical yield given as %ee; | 94% |
-
-
33125-05-2
Boc-D-Phg-OH

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid for 0.333333h; | 89% |
| With hydrogenchloride; Dowex 50*8 1.) THF, room temperature, 2 h.; Yield given. Multistep reaction; |
-
-
551-16-6
6-Aminopenicillanic Acid

-
-
700-63-0, 6485-52-5, 6485-67-2, 58429-87-1
(R)-phenylglycine amide

-
A
-
875-74-1
(R)-phenylglycine

-
B
-
69-53-4
ampicillin

| Conditions | Yield |
|---|---|
| With A. faecalis; immobilised penicillin acylase (EC 3.5.1.11) In water; acetonitrile at 0℃; Product distribution; Kinetics; Further Variations:; Solvents; reusing run; Enzymatic reaction; | A n/a B 86% |

-
-
186801-10-5
N-(2,4,6-trimethyl-benzenesulfonyl)-D-phenylglycine

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With sodium naphthalenide In 1,2-dimethoxyethane at -40℃; for 0.5h; | 78% |
-
-
188772-75-0
(R)-(furan-2-yl)(phenyl)methanamine

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With ozone In methanol at -78℃; for 0.25h; | 78% |
-
-
14257-84-2, 15962-46-6, 29633-99-6, 42429-20-9
N-acetyl-α-phenylglycine

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With water at 30℃; for 26h; D-aminoacylase from Streptomyces tuirus IFO 13418; | 74% |
-
-
611-71-2, 611-72-3, 17199-29-0, 90-64-2
MANDELIC ACID

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With D-glucose; ammonia; ammonium chloride In aq. phosphate buffer at 30℃; for 24h; pH=8; Catalytic behavior; Reagent/catalyst; Microbiological reaction; Green chemistry; Enzymatic reaction; enantioselective reaction; | 71% |
-
-
133545-89-8
[(1R)-2-(benzyloxy)-2-oxo-1-phenylethyl]ammonium 4-methyl-1-benzensulfonate

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With hydrogenchloride Heating; | 69% |
-
-
292638-84-7
styrene

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With D-glucose; oleic acid ethyl ester; ammonia; oxygen; ammonium chloride In aq. phosphate buffer at 30℃; for 24h; pH=8; Catalytic behavior; Reagent/catalyst; Microbiological reaction; Green chemistry; Enzymatic reaction; enantioselective reaction; | 62% |
| Multi-step reaction with 6 steps 1: styrene monooxygenase; NADPH; oxygen / Enzymatic reaction 2: epoxide hydrolase; water / Enzymatic reaction 3: alcohol dehydrogenase / Enzymatic reaction 4: aldehyde dehydrogenase; NAD / Enzymatic reaction 5: recombinant Escherichia coli expressing SMDH / aq. phosphate buffer / 0.5 h / 30 °C / pH 8 / Microbiological reaction; Enzymatic reaction 6: recombinant Escherichia coli DL39 (DE3) expressing DpgAT; L-glutamate / aq. phosphate buffer / 5 h / 30 °C / pH 8 / Microbiological reaction; Enzymatic reaction View Scheme |

| Conditions | Yield |
|---|---|
| With D-glucose; oleic acid ethyl ester; ammonia; ammonium chloride In aq. phosphate buffer at 30℃; for 24h; pH=8; Catalytic behavior; Reagent/catalyst; Microbiological reaction; Green chemistry; Enzymatic reaction; enantioselective reaction; | 53% |
| Multi-step reaction with 3 steps 1: phenylalanine ammonia lyase / Enzymatic reaction 2: pheylacrylic acid decarboxylase / Enzymatic reaction 3: ammonia; ammonium chloride; D-glucose; oleic acid ethyl ester; oxygen / aq. phosphate buffer / 24 h / 30 °C / pH 8 / Microbiological reaction; Green chemistry; Enzymatic reaction View Scheme | |
| Multi-step reaction with 8 steps 1: phenylalanine ammonia lyase / Enzymatic reaction 2: pheylacrylic acid decarboxylase / Enzymatic reaction 3: styrene monooxygenase; NADPH; oxygen / Enzymatic reaction 4: epoxide hydrolase; water / Enzymatic reaction 5: alcohol dehydrogenase / Enzymatic reaction 6: aldehyde dehydrogenase; NAD / Enzymatic reaction 7: recombinant Escherichia coli expressing SMDH / aq. phosphate buffer / 0.5 h / 30 °C / pH 8 / Microbiological reaction; Enzymatic reaction 8: recombinant Escherichia coli DL39 (DE3) expressing DpgAT; L-glutamate / aq. phosphate buffer / 5 h / 30 °C / pH 8 / Microbiological reaction; Enzymatic reaction View Scheme |
-
-
24461-61-8
(R)-Phenylglycine methyl ester

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 0℃; for 0.25h; Hydrolysis; | 35% |
-
-
4755-72-0, 39266-56-3
Chloro-phenyl-acetic acid

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With ammonia; benzonitrile | |
| With ammonia | |
| With ammonia; acetonitrile | |
| With n-heptan1ol; ammonia |
-
-
700-63-0, 6485-52-5, 6485-67-2, 58429-87-1
(R)-phenylglycine amide

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With hydrogen bromide | |
| Multi-step reaction with 2 steps 1: 42 percent / Alcaligenes faecalis penicillin acylase; potassium hydroxide / H2O / 0.28 h / 25 °C / pH 10 2: Alkaligenes faecalis penicillin acylase / 25 °C / pH 7.5 View Scheme |
-
-
10419-67-7
N-[(2S)-(benzoylamino)(phenyl)]ethanoic acid

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
60686-79-5
(R)-2-bromo-2-phenylacetic acid

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With ammonia; acetonitrile |
-
-
28290-68-8
(R)-(2-chloro-acetylamino)-phenyl-acetic acid

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
50859-90-0
(R)-D-mandeloylamino-phenyl-acetic acid

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With (1S)-10-camphorsulfonic acid |

| Conditions | Yield |
|---|---|
| With potassium phosphate buffer; Pseudomonas sp. AJ-11220 In various solvent(s) at 30℃; for 2h; pH 8.0; enzymatic reaction; | |
| With potassium phosphate buffer; Pseudomonas sp. AJ-11220 In various solvent(s) at 30℃; for 2h; |
-
-
25327-36-0
(Z)-2-(hydroxyimino)-2-phenylacetic acid

-
A
-
2935-35-5
(S)-2-phenylglycine

-
B
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With buffered acetic acid; strychnidin-10-one; mercury Product distribution; var. strychnine concentrations, other cathodic potentials (E): -1.05, -1.15, -1.30 V (SCE); object of study: optical yield; |
-
-
82264-50-4
N-carbamoyl-D,L-phenylglycine

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With potassium phosphate buffer; Pseudomonas sp. AJ-11220 In various solvent(s) at 30℃; for 2h; | |
| With potassium phosphate buffer; Pseudomonas sp. AJ-11220 In various solvent(s) at 30℃; for 2h; pH 7.5; enzymatic reaction; |

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With lithium hydroxide; ion exchange 1.) THF; Yield given. Multistep reaction; |
-
-
803648-97-7
Phenylglycine p-nitrophenyl ester

-
A
-
2935-35-5
(S)-2-phenylglycine

-
B
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With (R)-<methyl>pyridine; copper(II) ion In various solvent(s) at 25℃; Rate constant; enantioselectivity of cleavage; other chiral ligands; |
-
-
67-66-3
chloroform

-
-
100-52-7
benzaldehyde

-
A
-
2935-35-5
(S)-2-phenylglycine

-
B
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With potassium hydroxide; ammonia; (+)-(1S,2R)-N-hexadecyl-N-methylephedrinium bromide; lithium chloride In ethanol Product distribution; 5 deg C, 4 h, RT, overnight; asymmetric induction; |

-
-
119825-43-3
(R)-2-Amino-N-tert-butyl-2-phenyl-acetamide; hydrochloride

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 80℃; Yield given; |
-
-
111524-98-2
(2S)-2-phenyl-2-(tosylamino)acetic acid

-
A
-
2935-35-5
(S)-2-phenylglycine

-
B
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; samarium diiodide In tetrahydrofuran for 9h; Heating; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |
-
-
71849-99-5
(+/-)-α-phenylglycine hydrochloride

-
-
875-74-1
(R)-phenylglycine

| Conditions | Yield |
|---|---|
| With thionyl chloride for 2h; Heating; | 100% |
| With thionyl chloride In methanol at 20℃; Cooling with ice; | 99% |
| With thionyl chloride at 0 - 75℃; for 2h; | 98.9% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen; 5% Pd(II)/C(eggshell) In methanol; water under 1810.07 - 2068.65 Torr; for 4h; | 100% |
| With hydrogenchloride; hydrogen; 5%-palladium/activated carbon In methanol; water under 1810.07 - 2068.65 Torr; for 4h; | 100% |
| With hydrogenchloride; hydrogen; 5%-palladium/activated carbon In methanol; water under 1810.07 - 2068.65 Torr; for 4h; | 100% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tert-butyl alcohol at 20℃; for 1h; Inert atmosphere; | 100% |
| With potassium hydrogencarbonate In tetrahydrofuran; water for 18h; | 99% |
| With sodium hydroxide | 96% |
-
-
64-18-6
formic acid

-
-
875-74-1
(R)-phenylglycine

-
-
10419-71-3
(2R)-2-(N-formyl)amino-2-phenylacetic acid

| Conditions | Yield |
|---|---|
| With acetic anhydride at 20℃; for 1h; | 100% |
-
-
875-74-1
(R)-phenylglycine

-
-
2937-50-0
Allyl chloroformate

-
-
104128-14-5
(2R)-{[(allyloxy)carbonyl]amino}(phenyl)acetic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran at 0℃; for 0.25h; | 99% |
-
-
875-74-1
(R)-phenylglycine

-
-
1623-93-4
4-diphenylsulfonyl chloride

-
-
193808-16-1
N-(biphenyl-4-sulfonyl)-D-phenylglycine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In diethyl ether at 20℃; for 16h; | 99% |
-
-
2824-46-6
benzothiazole-2-sulfonyl chloride

-
-
875-74-1
(R)-phenylglycine

-
-
184222-92-2
(R)-(Benzothiazole-2-sulfonylamino)-phenyl-acetic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 10℃; for 10h; | 97% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid at 50℃; for 6.5h; Cooling with ice; | 96.8% |
| Stage #1: (R)-phenylglycine With sulfuric acid; nitric acid In water at 0℃; for 3h; Stage #2: With ammonia In water | 62% |
-
-
875-74-1
(R)-phenylglycine

-
-
60686-79-5
(R)-2-bromo-2-phenylacetic acid

| Conditions | Yield |
|---|---|
| With hydrogen bromide; sodium nitrite at 0℃; for 2h; | 96% |
| Stage #1: (R)-phenylglycine With sulfuric acid; potassium bromide In water Stage #2: With sodium nitrite In water at 0 - 20℃; for 2.5h; | |
| With sulfuric acid; potassium bromide; sodium nitrite In water |
-
-
85-44-9
phthalic anhydride

-
-
875-74-1
(R)-phenylglycine

-
-
15962-42-2
(R)-2-(1,3-dioxoisoindolin-2-yl)-2-phenylacetic acid

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 130℃; for 3h; Dean-Stark; | 96% |
| With acetic acid at 120℃; for 12h; Reflux; |
-
-
875-74-1
(R)-phenylglycine

-
-
14328-52-0
(R)-2-amino-2-cyclohexyl acetic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen In water; isopropyl alcohol at 50 - 60℃; for 6 - 8h; | 95.8% |
| With hydrogen; platinum(IV) oxide In acetic acid Ambient temperature; | 91% |
| With hydrogenchloride; hydrogen; rhodium under 38000 Torr; for 240h; | 79% |
| With hydrogenchloride; platinum at 70℃; Hydrogenation; | |
| With hydrogenchloride; hydrogen; platinum(IV) oxide In ethanol |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 0 - 20℃; Reflux; Inert atmosphere; | 95% |
| With hydrogenchloride | |
| With thionyl chloride at 20℃; |
-
-
875-74-1
(R)-phenylglycine

-
-
104-15-4
toluene-4-sulfonic acid

-
-
84860-34-4
L-(+)-α-Phenylglycine p-toluenesulfonate salt

| Conditions | Yield |
|---|---|
| In 1,2-dimethoxyethane; dichloromethane at 40℃; for 2h; | 95% |
-
-
875-74-1
(R)-phenylglycine

-
-
104-15-4
toluene-4-sulfonic acid

-
-
84860-33-3
D-(-)-α-Phenylglycine p-toluenosulfonate salt

| Conditions | Yield |
|---|---|
| In 1,2-dimethoxyethane; dichloromethane at 40℃; for 2h; | 95% |
-
-
875-74-1
(R)-phenylglycine

-
-
684-16-2
Hexafluoroacetone

-
-
202273-33-4
(R)-4-Phenyl-2,2-bis-trifluoromethyl-oxazolidin-5-one

| Conditions | Yield |
|---|---|
| 95% |
-
-
875-74-1
(R)-phenylglycine

-
-
104-15-4
toluene-4-sulfonic acid

-
-
100-51-6
benzyl alcohol

-
-
133545-89-8
[(1R)-2-(benzyloxy)-2-oxo-1-phenylethyl]ammonium 4-methyl-1-benzensulfonate

| Conditions | Yield |
|---|---|
| In cyclohexane; water for 4h; Dean-Stark; Reflux; | 95% |
| With water In toluene Heating; | 89% |

-
-
875-74-1
(R)-phenylglycine

-
-
103-72-0
phenyl isothiocyanate

-
-
1042303-80-9
(R)-phenyl-(3'-phenyl-thioureido)acetic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran; water at 20℃; for 24h; | 95% |
| Stage #1: (R)-phenylglycine With sodium hydroxide In tetrahydrofuran; water for 0.25h; Stage #2: phenyl isothiocyanate In tetrahydrofuran; water at 20℃; |
-
-
875-74-1
(R)-phenylglycine

-
-
56613-80-0
(R)-Phenylglycinol

| Conditions | Yield |
|---|---|
| Stage #1: (R)-phenylglycine With sodium tetrahydroborate; iodine In tetrahydrofuran at 64℃; for 18h; Stage #2: With methanol In tetrahydrofuran at 20℃; | 94% |
| With lithium borohydride; chloro-trimethyl-silane In tetrahydrofuran at 0 - 20℃; for 49h; | 93% |
| With lithium aluminium tetrahydride In tetrahydrofuran for 20h; Heating; | 92% |
-
-
875-74-1
(R)-phenylglycine

-
-
58632-95-4
2-(tert-Butoxycarbonyloxyimino)-2-phenylacetonitrile

-
-
33125-05-2
Boc-D-Phg-OH

| Conditions | Yield |
|---|---|
| With triethylamine In 1,4-dioxane; water for 18h; | 94% |
-
-
875-74-1
(R)-phenylglycine

-
-
23165-29-9
3,5-bistrifluoromethylphenylisothiocyanate

-
-
1042303-85-4
(R)-[3'-(3,5-bis-trifluoromethyl)phenyl-thioureido]-phenyl acetic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran; water at 20℃; for 24h; | 94% |
| Stage #1: 3,5-bistrifluoromethylphenylisothiocyanate With sodium hydroxide In tetrahydrofuran; water for 0.25h; Stage #2: (R)-phenylglycine In tetrahydrofuran; water at 20℃; |

| Conditions | Yield |
|---|---|
| With molecular sieve In methanol; ethanol under N2; aq. NaOH added to amino acid, soln. stirred (room temp., 5 min), soln. concd. (vac.), residue dried at 60°C (vac., 16 h), molecular sieves (4 Angstroem), ferrocenecarboxaldehyde and EtOH/MeOH added, mixt. stirred (room temp., 16 h); molecular sieves filtered off, filtrate concd. under vac., residue suspd. in pentane, solid filtered off, washed with pentane, dried under vac.; | 94% |

-
-
875-74-1
(R)-phenylglycine

-
-
108-24-7
acetic anhydride

-
-
14257-84-2, 15962-46-6, 29633-99-6, 42429-20-9
(R)-(-)-N-acetylphenylglycine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 0℃; for 6h; pH=11-12; Temperature; | 93.4% |
| Stage #1: (R)-phenylglycine With sodium hydroxide In water for 0.0333333h; Stage #2: acetic anhydride In water at 20℃; for 0.25h; Stage #3: With hydrogenchloride In water pH=1; | 80% |
| With sodium hydroxide In water at 0 - 5℃; pH=5; | 32% |
| With sodium hydroxide In water at 0 - 5℃; for 0.5h; | |
| In water at 70℃; for 0.5h; |
-
-
875-74-1
(R)-phenylglycine

-
-
39637-74-6
(1S)-(-)-camphanic chloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 288h; | 93% |

| Conditions | Yield |
|---|---|
| With pyridine; sodium hydroxide In acetone at 4℃; for 2h; | 93% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water at 80℃; for 0.00972222h; Substitution; microwave irradiation; | 93% |
-
-
383-63-1
ethyl trifluoroacetate,

-
-
875-74-1
(R)-phenylglycine

-
-
5042-71-7
(-)-(R)-N-trifluoroacetylphenylglycine

| Conditions | Yield |
|---|---|
| Stage #1: (R)-phenylglycine With N,N,N',N'-tetramethylguanidine In methanol for 0.0833333h; Stage #2: ethyl trifluoroacetate, In methanol at 20℃; for 30h; | 93% |
| With 1,1,3,3-tetramethylguanidine In methanol at 20℃; for 24h; | 93% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 0.166667h; | 92% |
| With sodium hydroxide | 88% |
| With sodium hydroxide at 0℃; for 1h; | 82% |
D-2-Phenylglycine Chemical Properties
IUPAC Name: (2R)-2-azaniumyl-2-phenylacetate
Empirical Formula: C8H9NO2
Molecular Weight: 151.1626g/mol
EINECS: 212-876-3
Structure of D-2-Phenylglycine (CAS NO.875-74-1):
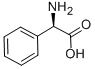
Index of Refraction: 1.589
Molar Refractivity: 40.9 cm3
Molar Volume: 121.3 cm3
Polarizability: 16.21×10-24cm3
Surface Tension: 57 dyne/cm
Density: 1.246 g/cm3
Flash Point: 128.4 °C
Enthalpy of Vaporization: 55.75 kJ/mol
Melting Point: 302 °C (dec.)(lit.)
Boiling Point: 288.7 °C at 760 mmHg
Vapour Pressure: 0.00107 mmHg at 25°C
Water Solubility: 0.3g/100mL
Product Categories: GLYCINESCAFFOLD;Pharmaceutical Intermediates;Amino ACIDS SERIES;Phenylglycine [Phg];Unusual Amino Acids;Amino Acids
Canonical SMILES: C1=CC=C(C=C1)C(C(=O)[O-])[NH3+]
Isomeric SMILES: C1=CC=C(C=C1)[C@H](C(=O)[O-])[NH3+]
InChI: InChI=1S/C8H9NO2/c9-7(8(10)11)6-4-2-1-3-5-6/h1-5,7H,9H2,(H,10,11)/t7-/m1/s1
InChIKey: ZGUNAGUHMKGQNY-SSDOTTSWSA-N
D-2-Phenylglycine Safety Profile
Hazard Codes:  T
T
Risk Statements: 23/24/25-36/37/38
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 22-24/25-45-36/37/39-26
S22:Do not breathe dust.
S24/25:Avoid contact with skin and eyes.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
WGK Germany: 3
HS Code: 29224995
D-2-Phenylglycine Specification
D-2-Phenylglycine , its cas register number is 875-74-1. It also can be called Benzeneacetic acid, alpha-amino-, (alphaR)- ;
D-(-)-alpha-Phenylglycine . D-2-Phenylglycine (CAS NO.875-74-1) is a white crystalline powder.
Related Products
- D-2-Phenylglycine
- 875753-08-5
- 87576-10-1
- 87576-94-1
- 875781-15-0
- 875781-17-2
- 875781-18-3
- 875781-43-4
- 875781-44-5
- 875781-45-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View