-
Name
Dibenzo-18-crown-6
- EINECS 238-041-3
- CAS No. 14187-32-7
- Article Data19
- CAS DataBase
- Density 1.108 g/cm3
- Solubility sparingly soluble in water
- Melting Point 162-164 °C(lit.)
- Formula C20H24O6
- Boiling Point 503.1 °C at 760 mmHg
- Molecular Weight 360.407
- Flash Point 206 °C
- Transport Information
- Appearance white to slightly beige fluffy powder
- Safety 26-36/37/39
- Risk Codes 36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, Xn
Xn
- Synonyms Dibenzo[b,k][1,4,7,10,13,16]hexaoxacyclooctadecane;NSC 147771;Dibenzo[b,k][1,4,7,10,13,16]hexaoxacyclooctadecin,6,7,9,10,17,18,20,21-octahydro-;Dibenzo-18-crown-6(6,7,9,10,17,18,20,21-Octahydrodibenzo);
- PSA 55.38000
- LogP 2.94880
Synthetic route

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide for 0.0833333h; Microwave irradiation; | 79% |
| With potassium carbonate In N,N-dimethyl-formamide for 24h; Reflux; | 21% |
| Stage #1: benzene-1,2-diol; 3-oxa-1,5-dichloropentane With sodium hydroxide In butan-1-ol at 90℃; for 2h; Heating; Stage #2: With sodium hydroxide for 16h; Reflux; Inert atmosphere; | 3.47% |
| With potassium carbonate In butan-1-ol at 115℃; | |
| With sodium hydroxide In dimethyl sulfoxide |
-
-
23116-94-1
bis(2-hydroxyphenoxyethyl)ether

-
-
111-44-4
3-oxa-1,5-dichloropentane

-
-
14187-32-7
dibenzo-18-crown-6

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide for 0.0833333h; Microwave irradiation; | 73% |
| With potassium carbonate In acetonitrile at 85℃; for 36h; | 69% |
| With sodium hydroxide 1.) n-BuOH, MeOH, 2.) n-BuOH, reflux, 17 h; Yield given. Multistep reaction; |
-
-
120-80-9
benzene-1,2-diol

-
-
111-44-4
3-oxa-1,5-dichloropentane

-
A
-
14187-32-7
dibenzo-18-crown-6

-
B
-
41758-00-3
1,2-bis[2'-(2''-chloroethoxy)ethoxy]benzene

-
C
-
111875-63-9
2-(5-chloro-3-oxa-1-pentyloxy)phenol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 90℃; for 2.5h; Yields of byproduct given; | A 55% B n/a C n/a |


-
-
14187-32-7
dibenzo-18-crown-6

| Conditions | Yield |
|---|---|
| With Iodine monochloride In benzene at 24.9℃; Equilibrium constant; |

| Conditions | Yield |
|---|---|
| In water at 25℃; Equilibrium constant; |

-
A
-
14187-32-7
dibenzo-18-crown-6

-
B
-
49563-87-3
1,4-diaza-bicyclo[2.2.2]octane dihydrochloride

| Conditions | Yield |
|---|---|
| In water at 25℃; Equilibrium constant; |
-
-
14187-32-7
dibenzo-18-crown-6

-
-
40100-11-6
4,4',5,5'-tetrabromodibenzo-18-crown-6 ether

| Conditions | Yield |
|---|---|
| With bromine; acetic acid In water Reflux; | 100% |
| With bromine; acetic acid for 5h; Heating; | 90% |
| With bromine; acetic acid at 95℃; for 12h; Bromination; | 80% |
-
-
14187-32-7
dibenzo-18-crown-6

-
-
32082-46-5
20,25-dinitro-2,3,11,12-dibenzo-1,4,7,10,13,16-hexaoxacyclooctadeca-2,11-diene

| Conditions | Yield |
|---|---|
| With nitric acid; lanthanum(III) nitrate In acetonitrile at 80℃; for 0.5h; nitrates of oth. metals; | 100% |
| With nitric acid; acetic anhydride In chloroform; acetic acid at 50℃; for 5h; Reagent/catalyst; Temperature; |
-
-
14187-32-7
dibenzo-18-crown-6

-
-
61853-51-8
2,3,12,13-tetranitro-6,7,9,10,17,18,20,21-octahydro-5,8,11,16,19,22-hexaoxadibenzo[b,k]cyclooctadecene

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid In dichloromethane at 20℃; for 12h; | 100% |
| With nitric acid | 96% |
| With sulfuric acid; nitric acid In dichloromethane at 20℃; for 48h; | 96% |
-
-
109-99-9
tetrahydrofuran

-
-
14187-32-7
dibenzo-18-crown-6

| Conditions | Yield |
|---|---|
| With potassium borohydride for 528h; Heating; | 100% |
-
-
14187-32-7
dibenzo-18-crown-6

-
-
3179-10-0
1-methoxy-4-(2-nitro-vinyl)-benzene

| Conditions | Yield |
|---|---|
| With PPA for 1h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 60℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 60℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 60℃; for 1h; | 100% |
-
-
14187-32-7
dibenzo-18-crown-6

- [1,2,5]thiadiazolo[3,4-c][1,2,5]thiadiazole
-
55904-34-2
[1,2,5]thiadiazolo[3,4-c][1,2,5]thiadiazole

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; | 100% |
-
-
14187-32-7
dibenzo-18-crown-6

-
-
106013-47-2
1-selena-2,5-diaza-2,4-cyclopentadiene-3,4-dicarbonitrile

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; | 100% |
-
-
14187-32-7
dibenzo-18-crown-6

| Conditions | Yield |
|---|---|
| In methanol Ar-atmosphere; stirring; filtration, evapn. (40°C, crystn. on walls of the vessel); | 99% |
| In methanol Ar-atmosphere; stirring; filtration, pptn. on ether addn., filtration, washing (MeOH/ether = 1/2,then ether), drying (vac., 40°C); elem. anal.; | 87% |
-
-
109-99-9
tetrahydrofuran

-
-
14187-32-7
dibenzo-18-crown-6

-
-
927437-38-5
(borohydrido)(1,4,7,10,13,16-hexaoxa-2,3:11,12-dibenzocyclooctadeca-2,11-diene-κ(6)O)(tetrahydrofuran)potassium

| Conditions | Yield |
|---|---|
| In tetrahydrofuran an NMR tube charged with KBH4, dibenzo-18-crown-6 and THF, refluxed for 22 d; | 99% |

-
-
14187-32-7
dibenzo-18-crown-6

-
-
1356843-06-5
potassium tetrakis(allyl)gallate

-
-
1356843-08-7
[K(dibenzo-18-crown-6)][Ga(η1-allyl)4]

| Conditions | Yield |
|---|---|
| In tetrahydrofuran under Ar, Schlenk techniques; dibenzocrown in THF added to soln. of Ga compd. in THF; volatiles removed (reduced pressure), pentane added to oil, supernatant decanted, residue washed (pentane), solid dried (vac.); elem. anal., XRD; | 99% |
-
-
14187-32-7
dibenzo-18-crown-6

-
-
1253215-98-3
[Al(η1-allyl)3(THF)]

-
-
60647-46-3, 7329-38-6
allyl potassium

-
-
1320213-77-1
[K(dibenzo-18-crown-6][tetrakis(η1-allyl)aluminate]

| Conditions | Yield |
|---|---|
| In tetrahydrofuran under Ar, Schlenk technique; addn. of allylpotassium and dibenzo-18-crown-6 to soln. of Al complex in THF; evapn. under vac., oily residue washed with pentane, solid dried under vac.; elem. anal.; | 98% |


| Conditions | Yield |
|---|---|
| With potassium hydroxide at 60℃; for 1h; | 97.9% |
-
-
14187-32-7
dibenzo-18-crown-6

-
-
58109-40-3
diphenyliodonium hexafluorophosphate

| Conditions | Yield |
|---|---|
| In ethyl acetate for 0.0833333h; Reflux; | 97.6% |

| Conditions | Yield |
|---|---|
| for 1h; Ambient temperature; | 97% |

-
-
14187-32-7
dibenzo-18-crown-6

-
-
102-96-5
(2-nitroethenyl)benzene

| Conditions | Yield |
|---|---|
| With PPA for 2h; Heating; | 97% |

| Conditions | Yield |
|---|---|
| In dichloromethane Ar-atmosphere; molar ratio Nb2Cl10:crown=1:2, room temp.; elem. anal.; | 97% |
-
-
109-99-9
tetrahydrofuran

-
-
14187-32-7
dibenzo-18-crown-6

| Conditions | Yield |
|---|---|
| at 20℃; for 12h; Inert atmosphere; | 97% |


| Conditions | Yield |
|---|---|
| In nitromethane byproducts: AgCl; (Ar), mixt. of Ru complex and AgOTf (1:4) stirred for 1 h in CH3NO2, AgCl filtered off, treated with 2 equiv. of dibenzo-18-crown-6, refluxed for 8 h; filtered, evapd.(vac.), washed (CH2Cl2), dissolved (acetone), chromy.(Al2O3 - acetone/EtOH 1:1), concd., pptd.(Et2O), elem. anal.; | 96% |


| Conditions | Yield |
|---|---|
| With sulfuric acid; sodium perchlorate In chloroform Heating; or KClO4; | 95% |
-
-
14187-32-7
dibenzo-18-crown-6

-
-
108-24-7
acetic anhydride

-
-
67722-66-1
1,1'-(6,7,9,10,17,18,20,21-octahydrodibenzo[b,k]-[1,4,7,10,13,16]hexaoxacyclooctadecine-2,13-diyl)diethanone

| Conditions | Yield |
|---|---|
| With phosphoric acid at 60℃; for 4h; Temperature; | 95% |
| With acetic acid at 96 - 100℃; for 1h; | 87% |
-
-
14187-32-7
dibenzo-18-crown-6

| Conditions | Yield |
|---|---|
| In neat (no solvent) heated to 100°C; dibenzo-18-crown-6 added with stirring; stirred for 5-10 min;; washed (water); filtered; dried at room temp.; sublimated (vac.); elem. anal.;; | 95% |
-
-
14187-32-7
dibenzo-18-crown-6

| Conditions | Yield |
|---|---|
| In diethyl ether under inert atm.; dibenzo-18-crown-6 added to soln. of Zn(BH4)2 in diethyl ether, stirred for 10 h; filtered off, washed (diethyl ether); elem. anal., DTA; | 95% |
| In tetrahydrofuran |

| Conditions | Yield |
|---|---|
| In diethyl ether inert atmosphere; crown ether addn. to soln. of B-compd., stirring (10 h); ppt. filtration off, washing (diethyl ether), vacuum drying; elem. anal.; | 95% |
| In diethyl ether stirring (room temp., 10 h, pptn.); filtration, washing (ether), drying (vac., room temp., 20 h); elem. anal.; | 90% |
-
-
14187-32-7
dibenzo-18-crown-6

-
-
851625-22-4
[((t)Bu3SiO)Cr(μ-OSi(t)Bu3)2]Na * benzene

-
-
851625-24-6
[((t)Bu3SiO)3Cr][Na(dibenzo-18-crown-6)]

| Conditions | Yield |
|---|---|
| In benzene dibenzo-18-crown-6 added to a soln. of Cr complex; crystd. for 12 h; elem. anal.; | 95% |
-
-
14187-32-7
dibenzo-18-crown-6

-
-
110692-06-3
4,4'-dibromodibenzo-18-crown-6 ether

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In chloroform at 20℃; | 94% |
-
-
14187-32-7
dibenzo-18-crown-6

-
-
134403-52-4
2,13-Diiodo-6,7,9,10,17,18,20,21-octahydro-5,8,11,16,19,22-hexaoxa-dibenzo[a,j]cyclooctadecene

| Conditions | Yield |
|---|---|
| With sulfuric acid; dihydrogen peroxide; iodine In ethanol for 4h; Heating; | 94% |
-
-
14187-32-7
dibenzo-18-crown-6

| Conditions | Yield |
|---|---|
| In chloroform boiled for 1 h;; evapd. in air at room temp.; washed (H2O); filtered; dried; sublimated (vac.); elem. anal.;; | 94% |
Dibenzo-18-crown-6 Specification
The Dibenzo-18-crown-6, with the CAS registry number 14187-32-7, is also known as Dibenzo(b,k)(1,4,7,10,13,16)hexaoxacyclooctadecane. It belongs to the product categories of API intermediates; Crown Ethers; Functional Materials; Macrocycles for Host-Guest Chemistry. Its EINECS number is 238-041-3. This chemical's molecular formula is C20H24O6 and molecular weight is 360.40. What's more, its systematic name is 6,7,9,10,17,18,20,21-octahydrodibenzo[b,k][1,4,7,10,13,16]hexaoxacyclooctadecine. Its classification codes are: (1)Drug / Therapeutic Agent; (2)Skin / Eye Irritant. It is stable at common pressure and temperature, and it should be sealed and stored in a cool, ventilated and dry place. Moreover, it should be protected from oxides and light. This chemical is a benzannulated crown ether. It is related to the non-benzannulated 18-crown-6. It is used as phase transfer catalyst and coordination reagent.
Physical properties of Dibenzo-18-crown-6 are: (1)ACD/LogP: 1.84; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.84; (4)ACD/LogD (pH 7.4): 1.84; (5)ACD/BCF (pH 5.5): 14.63; (6)ACD/BCF (pH 7.4): 14.63; (7)ACD/KOC (pH 5.5): 237.5; (8)ACD/KOC (pH 7.4): 237.5; (9)#H bond acceptors: 6; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 55.38 Å2; (13)Index of Refraction: 1.5; (14)Molar Refractivity: 95.7 cm3; (15)Molar Volume: 325.1 cm3; (16)Polarizability: 37.94×10-24cm3; (17)Surface Tension: 39.3 dyne/cm; (18)Density: 1.108 g/cm3; (19)Flash Point: 206 °C; (20)Enthalpy of Vaporization: 74.31 kJ/mol; (21)Boiling Point: 503.1 °C at 760 mmHg; (22)Vapour Pressure: 9.33E-10 mmHg at 25°C.
Preparation: this chemical can be synthesized from catechol and bis(chloroethyl)ether.
.png)
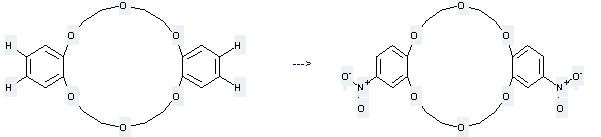
Uses of Dibenzo-18-crown-6: it can be used to produce cis-4,4'-Dinitrodibenzo-18-krone-6 at the temperature of 80 °C. It will need reagents 63% aq. HNO3, La(NO3)3·6H2O and solvent acetonitrile with the reaction time of 30 min. The yield is about 100%.
When you are using this chemical, please be cautious about it as the following:
This chemical is harmful by inhalation, in contact with skin and if swallowed. It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing, gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)SMILES: O1c3c(OCCOCCOc2ccccc2OCCOCC1)cccc3
(2)Std. InChI: InChI=1S/C20H24O6/c1-2-6-18-17(5-1)23-13-9-21-11-15-25-19-7-3-4-8-20(19)26-16-12-22-10-14-24-18/h1-8H,9-16H2
(3)Std. InChIKey: YSSSPARMOAYJTE-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 430mg/kg (430mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 31(2), Pg. 48, 1987. | |
| mouse | LD50 | oral | 4500mg/kg (4500mg/kg) | BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 52(11), Pg. 72, 1987. |
| rabbit | LD50 | intraperitoneal | 430mg/kg (430mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 31(2), Pg. 48, 1987. | |
| rat | LD50 | intraperitoneal | 560mg/kg (560mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 31(2), Pg. 48, 1987. | |
| rat | LD50 | oral | 2600mg/kg (2600mg/kg) | BEHAVIORAL: TREMOR BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Drug and Chemical Toxicology. Vol. 8, Pg. 451, 1985. |
Related Products
- Dibenzo-18-crown-6
- 14188-94-4
- 141892-41-3
- 141895-35-4
- 14189-82-3
- 141899-12-9
- 14190-16-0
- 141-90-2
- 14190-59-1
- 141-91-3
- 141914-99-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View