-
Name
Dicyclohexylcarbodiimide
- EINECS 208-704-1
- CAS No. 538-75-0
- Article Data104
- CAS DataBase
- Density 1.06 g/cm3
- Solubility reaction with water
- Melting Point 34-35 °C(lit.)
- Formula C13H22N2
- Boiling Point 277 °C at 760 mmHg
- Molecular Weight 206.331
- Flash Point 113.1 °C
- Transport Information
- Appearance colorless solid
- Safety 26-36/37/39-45-41-24-37/39-24/25-36-16-53
- Risk Codes 23/24/25-34-40-43-41-36/38-21-24-22-62-37/38-10-61
-
Molecular Structure
-
Hazard Symbols
 T,
T,  Xn
Xn
- Synonyms Carbodiimide,dicyclohexyl- (6CI,7CI,8CI);1,3-Dicyclohexylcarbodiimide;Bis(cyclohexyl)carbodiimide;DCC;DCCD;DCCI;Cyclohexanamine,N,N'-methanetetraylbis-;N,N'-Dicyclohexylcarbodiimide;NSC30022;NSC 53373;NSC 57182;N, N’-Dicyclohexylcarbodiimide (DCC);
- PSA 24.72000
- LogP 3.82570
Synthetic route

| Conditions | Yield |
|---|---|
| With dichloromethylenedimethyliminium chloride; triethylamine In dichloromethane at 0℃; | 100% |
| With bis(trichloromethyl) carbonate In 1-methyl-pyrrolidin-2-one at 30 - 40℃; for 6h; Autoclave; | 99.5% |
| With pyridine; chloroacetyl chloride In dichloromethane at 0 - 60℃; for 10h; Solvent; Temperature; Reagent/catalyst; | 97.3% |

| Conditions | Yield |
|---|---|
| With dicarbonyl(cyclopentadienyl)methyliron(II) In tetrahydrofuran at 60℃; for 24h; Reagent/catalyst; Inert atmosphere; | 99% |
| With dicarbonyl(cyclopentadienyl)methyliron(II); Triethoxysilane In tetrahydrofuran at 60℃; for 24h; Schlenk technique; Inert atmosphere; | 95% |
| With 1,1'-Thiocarbonyldi-2(1H)-pyridone In toluene for 8h; Heating; | 94% |
-
-
114316-64-2
N,N'-Dicyclohexyl-C-chlor-formamidinium

-
-
538-75-0
dicyclohexyl-carbodiimide

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water | 92.4% |

| Conditions | Yield |
|---|---|
| O=P(MeNCH2CH2)3N at 200 - 230℃; for 91h; | 92% |
| at 20 - 230℃; for 4h; Product distribution / selectivity; Molecular sieve; | 30.8% |
| [C5H5Fe(CO)2]2 In xylene for 24h; Heating; | 27% |

| Conditions | Yield |
|---|---|
| With di-2-pyridyl thionocarbonate; dmap In acetonitrile at 80℃; for 15h; | A n/a B 84% |
-
-
2387-23-7
1,3-Dicyclohexylurea

-
-
603-35-0
triphenylphosphine

-
A
-
538-75-0
dicyclohexyl-carbodiimide

-
B
-
791-28-6
Triphenylphosphine oxide

| Conditions | Yield |
|---|---|
| With aluminum oxide; LutClO4 constant current electrolysis; | A 72% B n/a |
| With aluminum oxide; LutClO4 In dichloromethane constant current electrolysis; | A 72% B n/a |

-
-
1212-29-9
1,3-dicyclohexylthiourea

-
-
603-35-0
triphenylphosphine

-
A
-
538-75-0
dicyclohexyl-carbodiimide

-
B
-
3878-45-3
triphenylphosphine sulfide

| Conditions | Yield |
|---|---|
| With aluminum oxide; LutClO4 In dichloromethane constant current electrolysis; | A 56% B n/a |

-
-
1212-29-9
1,3-dicyclohexylthiourea

-
-
603-35-0
triphenylphosphine

-
A
-
538-75-0
dicyclohexyl-carbodiimide

-
B
-
791-28-6
Triphenylphosphine oxide

| Conditions | Yield |
|---|---|
| With aluminum oxide; LutClO4 In dichloromethane constant current electrolysis; | A 56% B n/a |

-
-
2387-23-7
1,3-Dicyclohexylurea

-
A
-
931-53-3
Cyclohexyl isocyanide

-
B
-
2666-80-0
N-cyclohexylimidocarbonyl dichloride

-
C
-
538-75-0
dicyclohexyl-carbodiimide

| Conditions | Yield |
|---|---|
| With sodium hydroxide; triethylamine In chloroform for 4h; Heating; | A 21% B 38% C 18% |

-
-
931-53-3
Cyclohexyl isocyanide

-
-
108-91-8
cyclohexylamine

-
A
-
2387-23-7
1,3-Dicyclohexylurea

-
B
-
538-75-0
dicyclohexyl-carbodiimide

| Conditions | Yield |
|---|---|
| With oxygen; sodium carbonate; palladium diacetate In acetonitrile at 100℃; under 2068.6 Torr; for 3h; | A 10 % Chromat. B 35% |


| Conditions | Yield |
|---|---|
| With tetra-(n-butyl)ammonium iodide In acetonitrile at 50℃; for 12h; Inert atmosphere; Electrochemical reaction; | 26% |
| With iodine; oxygen; sodium carbonate; palladium diacetate In acetonitrile at 100℃; under 2068.6 Torr; for 3h; | 67 % Chromat. |
| With air; gold In hexane at 20℃; for 0.166667h; Kinetics; | |
| With Cumene hydroperoxide; iodine In tert-butyl methyl ether at 55℃; for 6h; | 51 %Chromat. |
-
-
3173-53-3
Cyclohexyl isocyanate

-
-
24901-29-9, 32721-26-9, 32721-27-0, 54932-28-4, 57664-94-5
3-methyl-1-phenylphospholane

-
-
538-75-0
dicyclohexyl-carbodiimide

| Conditions | Yield |
|---|---|
| With xylene |
-
-
2387-23-7
1,3-Dicyclohexylurea

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
538-75-0
dicyclohexyl-carbodiimide

| Conditions | Yield |
|---|---|
| With pyridine |

| Conditions | Yield |
|---|---|
| In dichloromethane |

-
-
83599-89-7
C29H41N2O2PS2

-
B
-
538-75-0
dicyclohexyl-carbodiimide

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; Rate constant; Product distribution; equilibrium studies, effect of addition of excess base or acid on the rate constants, effect of addition of triethylamine in various concentrations on the rate constants; |


| Conditions | Yield |
|---|---|
| In dichloromethane Mechanism; | |
| In dichloromethane |

-
-
92366-10-4
C25H33N2O2PS2

-
A
-
55979-88-9
O,O-diphenyl dithiophosphate

-
B
-
538-75-0
dicyclohexyl-carbodiimide

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; Rate constant; Product distribution; equilibrium studies, effect of addition of excess base or acid on the rate constants; |

-
-
92366-11-5
C37H57N2O2PS2

-
B
-
538-75-0
dicyclohexyl-carbodiimide

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; Rate constant; Product distribution; equilibrium studies, effect of addition of excess base or acid on the rate constants, effect of the initial substrate concentration on the rate constants; |


-
A
-
83599-89-7
C29H41N2O2PS2

-
C
-
538-75-0
dicyclohexyl-carbodiimide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; Product distribution; Rate constant; further amines, effect of base:substrate ratio on the product formation; |


| Conditions | Yield |
|---|---|
| With silica gel 1.) CH2Cl2, controlled potential electrolysis, 2.) petroleum ether, ether, 200 deg C; Yield given. Multistep reaction; |
-
-
34656-93-4
N,N'-dicyclohexylselenourea

-
-
538-75-0
dicyclohexyl-carbodiimide

| Conditions | Yield |
|---|---|
| With oxygen; 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran for 5h; Heating; Yield given; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Se, DBU / tetrahydrofuran / 1 h / Heating 2: DBU, O2 / tetrahydrofuran / 5 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 3-methyl-butan-1-ol 2: pyridine View Scheme |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 3h; Inert atmosphere; |


-
-
538-75-0
dicyclohexyl-carbodiimide

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran |

| Conditions | Yield |
|---|---|
| With triphenylphosphine In acetonitrile at 20℃; |

| Conditions | Yield |
|---|---|
| In water at 30℃; |
-
-
1112-38-5
O,O-dimethyl S-hydrogen phosphorothioate

-
-
538-75-0
dicyclohexyl-carbodiimide

-
-
87763-29-9
N-Dimethylphosphoryl-N,N'-dicyclohexylthiourea

| Conditions | Yield |
|---|---|
| In diethyl ether Product distribution; Ambient temperature; other phosphoro acids investigated; | 100% |
| In diethyl ether Ambient temperature; |

| Conditions | Yield |
|---|---|
| In tetrachloromethane Heating; | 100% |

| Conditions | Yield |
|---|---|
| In tetrachloromethane Heating; | 100% |

| Conditions | Yield |
|---|---|
| In tetrachloromethane Heating; | 100% |
-
-
45734-11-0
2-hydroxy-2-thiono-5,5-dimethyl-1,3,2-dioxaphosphorinane

-
-
538-75-0
dicyclohexyl-carbodiimide

-
A
-
15762-04-6
5,5-dimethyl-2-oxo-1,3,2-dioxaphosphorinan-2-yl 5,5-dimethyl-2-thioxo-1,3,2-dioxaphosphorinan-2-yl-oxide

-
B
-
1212-29-9
1,3-dicyclohexylthiourea

| Conditions | Yield |
|---|---|
| In acetonitrile Mechanism; Product distribution; Ambient temperature; | A 100% B 98% |

-
-
126621-87-2
3-(acetoxymethyl)-7α-chloro-Δ3-cephem-4-carboxylic acid 1,1-dioxide

-
-
538-75-0
dicyclohexyl-carbodiimide

-
-
129441-69-6, 129519-60-4, 148153-25-7
3-acetoxymethyl-7α-chloro-4-spiro-<3'-(1'-cyclohexyl-4'-cyclohexylimino-2'-oxoazetidinyl)>-2-cephem 1,1-dioxide

| Conditions | Yield |
|---|---|
| In dichloromethane for 0.0833333h; Product distribution; Mechanism; various cephalosporanic acid sulphones; | 100% |
| In dichloromethane for 0.0833333h; | 100% |
-
-
538-75-0
dicyclohexyl-carbodiimide

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride In tetrachloromethane Heating; | 100% |
-
-
538-75-0
dicyclohexyl-carbodiimide

-
-
627892-65-3
p-chlorobenzoic p-nitrophenyldiazoacetic anhydride

| Conditions | Yield |
|---|---|
| dirhodium tetraacetate In benzene | 100% |

| Conditions | Yield |
|---|---|
| With 2,4,6-trimethyl-pyridine In acetonitrile at 20℃; | 100% |
-
-
538-75-0
dicyclohexyl-carbodiimide

-
-
53959-22-1
(C6H11NH)2CN2C(C6H11NH)2

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In tetrahydrofuran at 20℃; for 48h; | 100% |

| Conditions | Yield |
|---|---|
| In 2-methyl-propan-1-ol; water for 15h; Heating; | 100% |


-
-
538-75-0
dicyclohexyl-carbodiimide

| Conditions | Yield |
|---|---|
| In benzene-d6 byproducts: Me3SiN=C=NSiMe3; (inert gas); an NMR tube charged with complex, 1,4-dimethoxybenzene and C6D6; carbodiimide added; not isolated, detected by NMR; | 100% |

| Conditions | Yield |
|---|---|
| With thymidine 5'-phosphate In dichloromethane at 20℃; | 100% |
| With 2,4,6-trimethyl-pyridine In dichloromethane at 20℃; | 98% |
-
-
421-85-2
Trifluoromethanesulfonamide

-
-
538-75-0
dicyclohexyl-carbodiimide

-
-
1338440-24-6
N-[bis(cyclohexylamino)methylidene]-1,1,1-trifluoromethanesulfonamide

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 6h; | 100% |
| In dichloromethane for 6h; | 100% |
| With nitromethane; copper dichloride for 2h; Milling; Green chemistry; | 98% |
-
-
27018-76-4
1-benzylindole-3-carboxylic acid

-
-
538-75-0
dicyclohexyl-carbodiimide

-
-
1363386-89-3
2-(1-benzyl-1H-indole-3-carbonyl)-1,3-dicyclohexylisourea

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 20℃; for 1.5h; | 100% |
-
-
112-80-1
cis-Octadecenoic acid

-
-
167407-91-2
N3,N6-di(tert-butoxycarbonyl)-3,6-diazaoctane-1,8-diol

-
-
538-75-0
dicyclohexyl-carbodiimide

-
A
-
167951-93-1
N,N'-di-tert-butylcarbonyl-N,N'-bis(2-hydroxyethyl)ethylenediamine dioleate

-
B
-
2387-23-7
1,3-Dicyclohexylurea

| Conditions | Yield |
|---|---|
| dmap In n-heptane at 20 - 40℃; Product distribution / selectivity; | A n/a B 99.6% |

-
-
95-51-2
2-Chloroaniline

-
-
538-75-0
dicyclohexyl-carbodiimide

-
-
96405-47-9
N-o-chlorophenyl-N', N"-dicyclohexylguanidine

| Conditions | Yield |
|---|---|
| With Sm[N(TMS)2]2(THF)3 at 20℃; for 0.25h; Inert atmosphere; | 99.3% |
| Stage #1: o-chloroaniline With C76H124N6Nd2O6Si4 In neat (no solvent) for 0.166667h; Schlenk technique; Inert atmosphere; Stage #2: dicyclohexyl-carbodiimide In neat (no solvent) at 60℃; for 0.25h; Schlenk technique; Inert atmosphere; | 98% |
| With C114H132N6O6Yb3*2C7H8 at 60℃; for 0.25h; Schlenk technique; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: dicyclohexyl-carbodiimide With iron(II) tetrafluoroborate hexahydrate; phenylsilane; tris(2-diphenylphosphinoethyl)phosphine In tetrahydrofuran at 100℃; for 20h; Stage #2: With water Reagent/catalyst; | 99% |
| With water; 5-methoxy-1,3,4-triphenyl-4,5-dihydro-1H-1,2-4-triazoline In 1,2-dimethoxyethane at 150℃; under 760.051 Torr; Inert atmosphere; Sealed tube; Microwave irradiation; | 75% |
| With sodium tetrahydroborate In isopropyl alcohol | |
| With hydrogen; palladium dihydroxide; barium sulfate In ethanol |
-
-
7343-26-2
carboxymethyltriphenylphosphoniumm chloride

-
-
538-75-0
dicyclohexyl-carbodiimide

-
-
80304-21-8
(1,3-dicyclohexylureidocarbonylmethyl)triphenylphosphonium chloride

| Conditions | Yield |
|---|---|
| In dichloromethane for 0.0833333h; | 99% |
-
-
108-91-8
cyclohexylamine

-
-
538-75-0
dicyclohexyl-carbodiimide

-
-
4833-41-4
N,N',N''-tricyclohexylguanidine

| Conditions | Yield |
|---|---|
| With C24H50N5Si2Y In neat (no solvent) at 20℃; for 2h; Inert atmosphere; Schlenk technique; | 99% |
| With zinc(II) oxide In toluene at 80℃; for 8h; | 95% |
| In tert-butyl alcohol at 100℃; for 24h; | 74% |
| With Zn-Al hydrotalcite In toluene at 110℃; for 12h; Sealed tube; | 52% |
| In hexane at 90℃; for 48h; Inert atmosphere; Pressure tube; |
-
-
538-75-0
dicyclohexyl-carbodiimide

-
-
829-85-6
diphenylphosphane

-
-
643014-87-3, 810692-09-2
(Z)-N,N'-dicyclohexyl(diphenylphosphino)formamidine

| Conditions | Yield |
|---|---|
| potassium hexamethylsilazane In tetrahydrofuran at 20℃; for 0.0833333h; | 99% |
| With n-butyllithium In tetrahydrofuran; hexane at 0 - 20℃; | 71% |

| Conditions | Yield |
|---|---|
| In hexane for 12h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| [Y((CH3)4C5Si(CH3)2N(phenyl))(trimethylsilylmethyl)(tetrahydrofuran)2] In benzene at 80℃; for 0.5h; | 99% |
| [{Me2Si(C5Me4)(NPh)}Y(CH2SiMe3)(thf)2] In benzene at 80℃; for 0.5h; |
-
-
24544-04-5
2,6-diisopropylbenzenamine

-
-
538-75-0
dicyclohexyl-carbodiimide

| Conditions | Yield |
|---|---|
| [(Me3Si)2N]3Yb(μ-Cl)Li(THF)3 In tetrahydrofuran at 60℃; for 24h; | 99% |
| With (C9H6CMe2CH2C5H4N-α)Y[N(SiHMe2)2]2 In toluene at 80℃; for 24h; Temperature; Schlenk technique; Glovebox; Inert atmosphere; | 90% |
| With [Ph2B(tBuIm)2FeNDipp][K(18-C-6)THF2] In tetrahydrofuran at 20℃; for 24h; Inert atmosphere; | 83% |
| With bis(tetrahydrofuran)calcium-bis[bis(trimethylsilyl)amide] In hexane at 80℃; for 2h; | 55% |
| With [Cp*2(Me)Zr(μ-O)Zr(NMe2)2(μ-O)Zr(Me)Cp*2] In benzene-d6 at 80℃; for 7.3h; Temperature; Reagent/catalyst; Time; Inert atmosphere; |
-
-
538-75-0
dicyclohexyl-carbodiimide

-
-
106-50-3
1,4-phenylenediamine

-
-
5012-73-7
2',2'-(1,4-phenylene)bis(1,3-dicyclohexylguanidine)

| Conditions | Yield |
|---|---|
| With diethylzinc In toluene at 50℃; for 2h; | 99% |
| [(Me3Si)2N]3Yb(μ-Cl)Li(THF)3 In tetrahydrofuran at 60℃; for 4h; | 96% |
| With [Li(THF)(DME)]3La[μ-η2η1(iPrN)2C(NC6H4p-Cl)]3 at 50℃; for 5h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| [(Me3Si)2N]3Yb(μ-Cl)Li(THF)3 In dichloromethane at 40℃; for 4h; | 99% |
| With C23H40AlN In toluene at 25℃; for 1h; Inert atmosphere; Schlenk technique; | 92% |
| With [Cp*2(Me)Zr(μ-O)Zr(NMe2)2(μ-O)Zr(Me)Cp*2] In benzene-d6 at 110℃; for 12h; Temperature; Reagent/catalyst; Time; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With C24H34Al2N4 In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; Schlenk technique; | 99% |
| With C36H52N5O4Si2Yb In neat at 60℃; for 6h; Inert atmosphere; | 97% |
| With [(1,2-bis[(2,6-diisopropylphenyl)imino]acenaphthene)Ga—Ga(1,2-bis[(2,6-diisopropylphenyl)imino]acenaphthene)] In benzene-d6 at 60℃; for 1.3h; Temperature; Schlenk technique; Sealed tube; | 97% |
| [(Me3Si)2N]3Yb(μ-Cl)Li(THF)3 In tetrahydrofuran at 60℃; for 12h; | 96% |
| Stage #1: dicyclohexyl-carbodiimide In neat (no solvent) at 80℃; for 0.166667h; Stage #2: 1-amino-naphthalene In neat (no solvent) at 80℃; | 61% |

| Conditions | Yield |
|---|---|
| With Sm[N(TMS)2]2(THF)3 at 20℃; for 0.166667h; Inert atmosphere; | 99% |
| With [Li(THF)(DME)]3La[μ-η2η1(iPrN)2C(NC6H4p-Cl)]3 at 25℃; for 0.5h; Inert atmosphere; | 99% |
| With (Me3SiNC(Ph)N(CH2)3NC(Ph)NSiMe3)Y[O2,6-(tBu)2-4-(Me)C6H2](DME) at 60℃; for 0.5h; Inert atmosphere; Schlenk technique; | 99% |
Dicyclohexylcarbodiimide Specification
1.Introduction of Dicyclohexylcarbodiimide
Dicyclohexylcarbodiimide,with its CAS NO.538-75-0,is a kind of white or slight yellow crystalline solid. It has synonyms of 1,3-Dicyclohexylcarbodiimide ; Bis(cyclohexyl)carbodiimide ; Carbodicyclohexylimide ; DCC ; DCCD ; DCCI ; N,N'-Dicyclohexylcarbodiimide ; N,N'-Methanetetraylbiscyclohexaamine and NSC 30022. Dicyclohexylcarbodiimide is an imide. Amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx). Dicyclohexylcarbodiimide is incompatible with acids and oxidizing agents. It is probably combustible.
2.Properties of Dicyclohexylcarbodiimide
(1) Molecular Weight 206.32718 [g/mol] (2) Molecular Formula C13H22N2 (3) XLogP3-AA 4.7
(4) H-Bond Acceptor 2 (5) Rotatable Bond Count 2 (6) Exact Mass 206.178299
(7) MonoIsotopic Mass 206.178299 (8) Topological Polar Surface Area 24.7 (9) Heavy Atom Count 15
(10) Complexity 201 (11) Covalently-Bonded Unit Count 1 (12) Feature 3D Acceptor Count 2
(13) Feature 3D Cation Count 1 (14) Feature 3D Ring Count 2 (15) Effective Rotor Count 4.4
(16) Conformer Sampling RMSD 0.6 (17) CID Conformer Count 23
3.Structure descriptors of Dicyclohexylcarbodiimide
Canonical SMILES: C1CCC(CC1)N=C=NC2CCCCC2
InChI: InChI=1S/C13H22N2/c1-3-7-12(8-4-1)14-11-15-13-9-5-2-6-10-13/h12-13H,1-10H2
InChIKey: QOSSAOTZNIDXMA-UHFFFAOYSA-N
4.Safety information of Dicyclohexylcarbodiimide
Hazard Codes:  T,
T, Xn
Xn
Risk Statements: 23/24/25-34-40-43-41-36/38-21-24-22-62-37/38-10-61
R23/24/25: Toxic by inhalation, in contact with skin and if swallowed
R34: Causes burns
R40: Limited evidence of a carcinogenic effect
R43: May cause sensitization by skin contact
R41: Risk of serious damage to eyes
R36/38: Irritating to eyes and skin
R21: Harmful in contact with skin
R24: Toxic in contact with skin
R22: Harmful if swallowed
R62: Possible risk of impaired fertility
R37/38: Irritating to respiratory system and skin
R10: Flammable
R61: May cause harm to the unborn child
Safety Statements: 26-36/37/39-45-41-24-37/39-24/25-36-16-53
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection
S45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
S41: In case of fire and/or explosion do not breathe fumes
S24: Avoid contact with skin
S37/39: Wear suitable gloves and eye/face protection
S24/25: Avoid contact with skin and eyes
S36: Wear suitable protective clothing
S16: Keep away from sources of ignition - No smoking
S53: Avoid exposure - obtain special instruction before use
RIDADR: UN 2922 8/PG 2
WGK Germany: 3
RTECS: FF2160000
F: 3-8-10-21: Hygroscopic. Photosensitive. Keep under argon. Sensitive to humidity.
HazardClass: 6.1
PackingGroup: II
5.Production of Dicyclohexylcarbodiimide
Dicyclohexylcarbodiimide has several synthesis methods. Pri-Bara et al. use palladium acetate, iodine, and oxygen to couple cyclohexyl amine and cyclohexyl isocyanide.
C6H11NC + C6H11NH2 + O2 → (C6H11N)2C + H2O
Tang et al. condense two isocyanates using the catalyst OP(MeNCH2CH2)3N in yields of 92%:
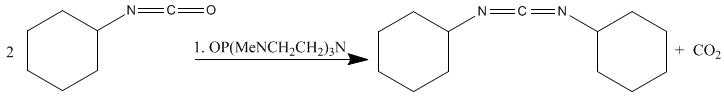
Dicyclohexylcarbodiimide has also been made from dicyclohexylurea using a phase transfer catalyst by Jaszay et al. The disubstituted urea, arenesulfonyl chloride, and potassium carbonate react in toluene in the presence of benzyl trimethylammonium chloride to give Dicyclohexylcarbodiimide in 50% yield.

6.Toxity data of Dicyclohexylcarbodiimide
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | skin | 10mL/kg (10mL/kg) | National Technical Information Service. Vol. OTS0555962, | |
| mouse | LD50 | intraperitoneal | > 800mg/kg (800mg/kg) | BEHAVIORAL: MUSCLE WEAKNESS SKIN AND APPENDAGES (SKIN): HAIR: OTHER | National Technical Information Service. Vol. OTS0555962, |
| mouse | LD50 | oral | > 800mg/kg (800mg/kg) | BEHAVIORAL: MUSCLE WEAKNESS SKIN AND APPENDAGES (SKIN): HAIR: OTHER | National Technical Information Service. Vol. OTS0555962, |
| rat | LC50 | inhalation | 159mg/m3/6H (159mg/m3) | SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE VASCULAR: REGIONAL OR GENERAL ARTERIOLAR OR VENOUS DILATION SKIN AND APPENDAGES (SKIN): HAIR: OTHER | National Technical Information Service. Vol. OTS0555962, |
| rat | LD50 | intraperitoneal | 10mg/kg (10mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: DYSPNEA LUNGS, THORAX, OR RESPIRATION: CYANOSIS | National Technical Information Service. Vol. OTS0555962, |
| rat | LD50 | oral | 400mg/kg (400mg/kg) | BEHAVIORAL: MUSCLE WEAKNESS GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" SKIN AND APPENDAGES (SKIN): HAIR: OTHER | National Technical Information Service. Vol. OTS0555962, |
Related Products
- Dicyclohexylcarbodiimide
- 53878-47-0
- 53879-54-2
- 53880-05-0
- 53880-51-6
- 53880-86-7
- 538-81-8
- 53882-80-7
- 53882-81-8
- 5388-28-3
- 53882-91-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View