-
Name
Ethyl cyclopropanecarboxylate
- EINECS 225-010-4
- CAS No. 4606-07-9
- Article Data41
- CAS DataBase
- Density 1.063 g/cm3
- Solubility Immiscible with water
- Melting Point
- Formula C6H10O2
- Boiling Point 134 °C at 760 mmHg
- Molecular Weight 114.144
- Flash Point 29.7 °C
- Transport Information UN 3272 3/PG 2
- Appearance clear pale yellow liquid
- Safety 16
- Risk Codes 11
-
Molecular Structure
-
Hazard Symbols
 F
F
- Synonyms Ethyl cyclopropylcarboxylate;NSC 60696;
- PSA 26.30000
- LogP 0.95950
Synthetic route

| Conditions | Yield |
|---|---|
| With sulfuric acid In ethanol | 98% |
-
-
77100-87-9
ethyl α,γ-dichlorobutyrate

-
-
4606-07-9
ethyl cyclopropylcarboxylate

| Conditions | Yield |
|---|---|
| With tetraethylammonium tosylate In dimethyl sulfoxide electrolysis; | 82% |
-
-
2969-81-5
4-bromoethylbutanoate

-
A
-
999-10-0
ethyl 4-hydroxybutanoate

-
B
-
4606-07-9
ethyl cyclopropylcarboxylate

| Conditions | Yield |
|---|---|
| With oxygen; tetraethylammonium perchlorate In N,N-dimethyl-formamide at 20℃; electroreduction at -1.1 V; | A 10% B 68% |

| Conditions | Yield |
|---|---|
| Stage #1: n-octyne With n-butyllithium In tetrahydrofuran at 20℃; Stage #2: ethyl 4-iodobutyrate In tetrahydrofuran at 20℃; | A 9% B 65% |


| Conditions | Yield |
|---|---|
| With sodium tert-pentoxide; benzene | |
| With sodium tert-pentoxide In benzene | |
| With N-(diphenylmethylidene)phenylamine at -78℃; | |
| With copper(I) oxide; Cyclohexyl isocyanide | |
| With potassium hydroxide; tetrabutylammomium bromide In toluene at 40℃; for 2h; | 91 % Chromat. |
-
-
58539-11-0
3-Bromo-2-bromomethyl-propionic acid ethyl ester

-
-
4606-07-9
ethyl cyclopropylcarboxylate

| Conditions | Yield |
|---|---|
| With ethanol; zinc |
-
-
2969-81-5
4-bromoethylbutanoate

-
-
1655-07-8
ethyl 2-oxocyclohexane carboxylate

-
A
-
4606-07-9
ethyl cyclopropylcarboxylate

-
B
-
124355-49-3
ethyl 1-(3-(ethoxycarbonyl)propyl)-2-oxocyclohexanecarboxylate

| Conditions | Yield |
|---|---|
| With sodium ethanolate |


| Conditions | Yield |
|---|---|
| With sulfuric acid | |
| With ε-caprolactam methyl sulfonate at 30℃; for 8h; chemoselective reaction; | 87 %Chromat. |
| With quaternary ammonium salt functionalized methoxypolyethylene glycols-supported phosphotungstic acid catalyst at 50℃; for 3h; | |
| With thionyl chloride Reflux; | |
| With sulfuric acid for 12h; Inert atmosphere; Reflux; |
-
-
19999-37-2
Tosylat von 4-Aethoxy-butin-(3)-ol

-
-
4606-07-9
ethyl cyclopropylcarboxylate

| Conditions | Yield |
|---|---|
| With pyridine In water; acetone at 55℃; for 120h; |
-
-
75-21-8
oxirane

-
-
867-13-0
diethoxyphosphoryl-acetic acid ethyl ester

-
-
4606-07-9
ethyl cyclopropylcarboxylate

| Conditions | Yield |
|---|---|
| With 1.) base Multistep reaction; |
-
-
36847-51-5
ethyl 2,4-dibromobutyrate

-
A
-
2969-81-5
4-bromoethylbutanoate

-
B
-
4606-07-9
ethyl cyclopropylcarboxylate

-
C
-
105-54-4
butanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; Product distribution; Mechanism; electrolysis; I*, napp; various conc. of the ester; in the presence and in the absence of 3,4-xylenol; |

-
-
2969-81-5
4-bromoethylbutanoate

-
-
3978-80-1
Boc-Tyr-OH

-
A
-
4606-07-9
ethyl cyclopropylcarboxylate

-
B
-
171858-32-5
ethyl 4-<4'-<<2-<(tert-butoxycarbonyl)amino>-2-carboxy>ethyl>phenoxy>butanoate

| Conditions | Yield |
|---|---|
| With sodium hydride 1.) THF, RT, 5 min, 2.) THF, reflux, 21 h; Yield given. Multistep reaction; |


| Conditions | Yield |
|---|---|
| With 1-hydroxy-2(1H)-pyridinethione; dicyclohexyl-carbodiimide 1.) CHCl3, room temperature, 6 h, 2.) CHCl3, room temperature, irradiation; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With 2-mercaptopyridine-1-oxide sodium salt 2.) room temperature, irradiation; Multistep reaction; |
-
-
3153-36-4
4-chloro-butyric acid ethyl ester

-
A
-
96-48-0
4-butanolide

-
B
-
4606-07-9
ethyl cyclopropylcarboxylate

-
C
-
1759-53-1
cyclopropanecarboxylic acid

| Conditions | Yield |
|---|---|
| at 180℃; |


| Conditions | Yield |
|---|---|
| With potassium hydroxide; pumice stone at 180℃; |

| Conditions | Yield |
|---|---|
| With sulfuric acid |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 58 percent / magnesium iodide / toluene / 8 h / Heating 2: 1.) 1-hydroxy-pyridine-2-thione, dicyclohexyl-carbodiimide / 1.) CHCl3, room temperature, 6 h, 2.) CHCl3, room temperature, irradiation View Scheme |

| Conditions | Yield |
|---|---|
| With phenylhydrazine In toluene | |
| In ethyl acetate; toluene |

| Conditions | Yield |
|---|---|
| With chloro(1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene)gold(I); sodium tetrakis[(3,5-di-trifluoromethyl)phenyl]borate In dichloromethane at 40℃; under 6000.6 Torr; for 14h; Catalytic behavior; Temperature; Inert atmosphere; Schlenk technique; | 70 %Chromat. |
-
-
623-73-4
diazoacetic acid ethyl ester

-
-
74-85-1
ethene

-
A
-
4606-07-9
ethyl cyclopropylcarboxylate

-
B
-
623-91-6
diethyl Fumarate

-
C
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With tetrakis(trifluoroacetato)rhodium(II) In dichloromethane at 20℃; under 6000.6 Torr; for 14h; Reagent/catalyst; Inert atmosphere; Schlenk technique; | A 41 %Chromat. B n/a C n/a |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
74-85-1
ethene

-
A
-
4606-07-9
ethyl cyclopropylcarboxylate

-
-
729-87-3, 13950-16-8
(E)-Cyclopropan-1,2,3-tricarbonsaeuretriethylester

| Conditions | Yield |
|---|---|
| With chloro(1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene)gold(I); sodium tetrakis[(3,5-di-trifluoromethyl)phenyl]borate In dichloromethane at 20℃; under 1500.15 Torr; for 14h; Catalytic behavior; Pressure; Inert atmosphere; Schlenk technique; | A 39 %Chromat. B 35 %Chromat. |


| Conditions | Yield |
|---|---|
| With hydrazine hydrate at 120℃; for 16h; | 100% |
| With hydrazine hydrate | |
| With hydrazine In ethanol for 3h; Heating; | |
| With hydrazine hydrate at 80℃; | |
| With hydrazine hydrate In ethanol at 80℃; for 18h; Sealed tube; |
-
-
4606-07-9
ethyl cyclopropylcarboxylate

-
-
79506-64-2
2-((2-methoxyphenyl)thio)acetonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 2-((2-methoxyphenyl)thio)acetonitrile With n-butyllithium In hexane at -78℃; Stage #2: ethyl cyclopropylcarboxylate In hexane at -78 - 20℃; | 100% |
-
-
4606-07-9
ethyl cyclopropylcarboxylate

-
-
67132-62-1
dimethyl aluminum selenide

-
-
67132-66-5
Cyclopropanecarboselenoic acid Se-methyl ester

| Conditions | Yield |
|---|---|
| In dichloromethane; toluene 1.) 0 deg C, 2.) up to RT; | 96% |
-
-
4606-07-9
ethyl cyclopropylcarboxylate

-
-
71-43-2
benzene

-
-
17496-14-9
2,3-dihydro-2-methyl-1H-inden-1-one

| Conditions | Yield |
|---|---|
| aluminium trichloride for 16h; Heating; | 93% |

| Conditions | Yield |
|---|---|
| With magnesium In diethyl ether 1) room temp., 30 min, 2) reflux, 1 h; | 92.7% |
-
-
4606-07-9
ethyl cyclopropylcarboxylate

-
-
75-05-8
acetonitrile

-
-
118431-88-2
3-cyclopropyl-3-oxo-propionitrile

| Conditions | Yield |
|---|---|
| With sodium hydride In 1,4-dioxane; mineral oil at 20℃; for 16h; | 90% |
| With potassium tert-butylate; isopropyl alcohol In 2-methyltetrahydrofuran at 20℃; for 2h; Solvent; Time; Inert atmosphere; | 48% |
| With sodium hydride In tetrahydrofuran for 15h; Heating / reflux; |
-
-
4606-07-9
ethyl cyclopropylcarboxylate

-
-
451-82-1
(2-fluorophenyl)acetic acid

-
-
150322-73-9
1-cyclopropyl-2-(2-fluorophenyl)ethanone

| Conditions | Yield |
|---|---|
| With sodium hydride In dimethyl sulfoxide; toluene at 95 - 110℃; for 1.5h; Large scale; | 88% |
| Stage #1: (2-fluorophenyl)acetic acid With magnesium; isopropyl bromide In tetrahydrofuran; toluene at 15 - 65℃; Stage #2: ethyl cyclopropylcarboxylate In tetrahydrofuran; toluene at 5 - 65℃; |

| Conditions | Yield |
|---|---|
| With boron trifluoride In Hexadecane at 110℃; for 18h; Autoclave; | 88% |
-
-
108-86-1
bromobenzene

-
-
4606-07-9
ethyl cyclopropylcarboxylate

-
-
5785-66-0
cyclopropyl diphenyl carbinol

| Conditions | Yield |
|---|---|
| With magnesium; copper(II) oxide In tetrahydrofuran at 65℃; for 4h; Barbier Coupling Reaction; chemoselective reaction; | 84% |
| With magnesium; copper(II) oxide In tetrahydrofuran at 65℃; for 4h; chemoselective reaction; | 84% |
-
-
4606-07-9
ethyl cyclopropylcarboxylate

-
-
13633-36-8
N,N-bis(2-bromoallyl)-4-methylaniline

-
-
545435-10-7
3-(2-cyclopropyl-2-oxoethyl)-2,5-dihydro-4-methyl-1-(4-methylphenyl)-1H-pyrrole

| Conditions | Yield |
|---|---|
| Stage #1: N,N-bis(2-bromoallyl)-4-methylaniline With tert.-butyl lithium In diethyl ether; pentane at -78 - 20℃; for 1.5h; Stage #2: ethyl cyclopropylcarboxylate In diethyl ether; pentane at -78℃; for 1h; | 82% |

| Conditions | Yield |
|---|---|
| With chromium chloride; magnesium In tetrahydrofuran at 80℃; for 12h; Inert atmosphere; | 82% |
-
-
4606-07-9
ethyl cyclopropylcarboxylate

-
-
13633-36-8
N,N-bis(2-bromoallyl)-4-methylaniline

-
-
545435-11-8
5-cyclopropyl-1,2,3,4,5,6-hexahydro-2-(4-methylphenyl)cyclopenta[c]pyrrol-5-ol

| Conditions | Yield |
|---|---|
| Stage #1: N,N-bis(2-bromoallyl)-4-methylaniline With tert.-butyl lithium In diethyl ether; pentane at -78 - 20℃; for 1.5h; Stage #2: ethyl cyclopropylcarboxylate In diethyl ether; pentane at -78 - 20℃; | 80% |
-
-
106-38-7
para-bromotoluene

-
-
4606-07-9
ethyl cyclopropylcarboxylate

-
-
7035-46-3
(cyclopropyl)(di-p-tolyl)methanol

| Conditions | Yield |
|---|---|
| With magnesium; copper(II) oxide In tetrahydrofuran at 65℃; for 4h; Barbier Coupling Reaction; chemoselective reaction; | 78% |
| With magnesium; copper(II) oxide In tetrahydrofuran at 65℃; for 4h; chemoselective reaction; | 78% |
-
-
4606-07-9
ethyl cyclopropylcarboxylate

-
-
13170-43-9
(trimethylsilyl)methylmagnesium chloride

-
-
144729-87-3
2-cyclopropyl-2-propenyltrimethylsilane

| Conditions | Yield |
|---|---|
| With cesium chloride; silica gel | 73% |
-
-
109-72-8, 29786-93-4
n-butyllithium

-
-
201230-82-2
carbon monoxide

-
-
4606-07-9
ethyl cyclopropylcarboxylate

-
-
136201-68-8
1-Cyclopropyl-hexane-1,2-dione

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether; hexane; pentane a) -110 deg C, 2 h, b) to r.t.; | 67% |


| Conditions | Yield |
|---|---|
| aluminium trichloride In toluene Heating; | 67% |

| Conditions | Yield |
|---|---|
| Stage #1: 2,2'-(2-(2,4-dimethylphenyl)ethane-1,1-diyl)bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) With 2,2,6,6-tetramethylpiperidinyl-lithium In tetrahydrofuran; hexane at 0℃; for 0.25h; Inert atmosphere; Stage #2: ethyl cyclopropylcarboxylate In tetrahydrofuran; hexane at 50℃; for 0.25h; Inert atmosphere; Stage #3: methyl iodide In tetrahydrofuran; hexane at 20℃; for 0.25h; Inert atmosphere; regioselective reaction; | 65% |

-
-
4606-07-9
ethyl cyclopropylcarboxylate

-
-
142523-69-1
1-benzyloxy-2-iodobenzene

| Conditions | Yield |
|---|---|
| Stage #1: 1-benzyloxy-2-iodobenzene With tert.-butyl lithium In tetrahydrofuran; pentane at -78 - -25℃; Stage #2: ethyl cyclopropylcarboxylate In tetrahydrofuran; pentane at -78 - 20℃; Stage #3: With hydrogenchloride In tetrahydrofuran; water; pentane at 20℃; | 64% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; hydroxylamine hydrochloride In ethanol for 8h; Ambient temperature; | 60% |
| With hydroxylamine; sodium methylate | |
| With potassium hydroxide; hydroxylamine hydrochloride In methanol |
-
-
201230-82-2
carbon monoxide

-
-
4606-07-9
ethyl cyclopropylcarboxylate

-
-
594-19-4
tert.-butyl lithium

-
A
-
15963-60-7
1-cyclohexyl-2,2-dimethyl-1-propanone

-
B
-
34650-62-9
Cyclopropyl-1-dimethyl-3,3-butan-dion-1,2

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether; hexane; pentane a) -110 deg C, 2 h, b) to r.t.; | A 3% B 60% |


| Conditions | Yield |
|---|---|
| Stage #1: 2-chloro-4-methylpyrimidine With lithium hexamethyldisilazane In tetrahydrofuran at -78℃; for 1h; Stage #2: ethyl cyclopropylcarboxylate In tetrahydrofuran at -78℃; for 5h; | 46% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-(benzyloxy)-2-bromo-4-methylbenzene With tert.-butyl lithium In tetrahydrofuran; pentane at -78 - -25℃; Stage #2: ethyl cyclopropylcarboxylate In tetrahydrofuran; pentane at -78 - 20℃; Stage #3: With hydrogenchloride In tetrahydrofuran; water; pentane at 20℃; | 45% |
-
-
4606-07-9
ethyl cyclopropylcarboxylate

-
-
96010-06-9
(2-(4-bromophenyl)cyclopropane-1,1-diyl)dibenzene

-
-
1251909-11-1
cyclopropyl[bis-(4-(2,2-diphenylcyclopropyl)phenyl)]methanol

| Conditions | Yield |
|---|---|
| Stage #1: (2-(4-bromophenyl)cyclopropane-1,1-diyl)dibenzene With magnesium In tetrahydrofuran Stage #2: ethyl cyclopropylcarboxylate In tetrahydrofuran | 43% |
-
-
121393-39-3
5,10,15,20-tetra(2,4,6-trimethylphenyl)porphyrinate rhodium(II)

-
-
4606-07-9
ethyl cyclopropylcarboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 5,10,15,20-tetra(2,4,6-trimethylphenyl)porphyrinate rhodium(II) With triphenylphosphine In benzene at 25℃; for 0.166667h; Stage #2: ethyl cyclopropylcarboxylate In benzene at 25℃; for 96h; Inert atmosphere; Darkness; regioselective reaction; | 38% |

| Conditions | Yield |
|---|---|
| With triisopropoxytitanium(IV) chloride; cyclohexylmagnesiumchloride In tetrahydrofuran; diethyl ether at 20℃; for 2h; Inert atmosphere; | 33% |
Ethyl cyclopropanecarboxylate Specification
The CAS register number of Cyclopropanecarboxylicacid, ethyl ester is 4606-07-9. It also can be called as Ethylcyclopropanecarboxylate and the IUPAC name about this chemical is ethyl cyclopropanecarboxylate. The molecular formula about this chemical is C6H10O2 and the molecular weight is 114.14. It belongs to the following product categories, such as Cyclopropanes; Simple 3-Membered Ring Compounds; C6 to C7; Carbonyl Compounds; Esters and so on. This chemical is highly flammable. When you are using it, please keep away from sources of ignition.
Physical properties about Cyclopropanecarboxylicacid, ethyl ester are: (1)ACD/LogP: 1.08; (2)ACD/LogD (pH 5.5): 1.08; (3)ACD/LogD (pH 7.4): 1.08; (4)ACD/BCF (pH 5.5): 3.86; (5)ACD/BCF (pH 7.4): 3.86; (6)ACD/KOC (pH 5.5): 91.59; (7)ACD/KOC (pH 7.4): 91.59; (8)#H bond acceptors: 2; (9)#Freely Rotating Bonds: 3; (10)Polar Surface Area: 26.3Å2; (11)Index of Refraction: 1.46; (12)Molar Refractivity: 29.41 cm3; (13)Molar Volume: 107.3 cm3; (14)Polarizability: 11.66x10-24cm3; (15)Surface Tension: 36.6 dyne/cm; (16)Enthalpy of Vaporization: 37.15 kJ/mol; (17)Boiling Point: 134 °C at 760 mmHg; (18)Vapour Pressure: 8.25 mmHg at 25°C.
Preparation: this chemical can be prepared by 2,4-dichloro-butyric acid ethyl ester. This reaction will need reagent Et4NO3SC6H4Me-p and solvent dimethylsulfoξde. The reaction needs electrolysis. The yield is about 82% .
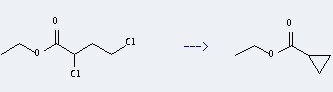
Uses of Cyclopropanecarboxylicacid, ethyl ester: it can be used to produce cyclopropanecarbohydroxamic acid. This reaction will need reagent sodium methylate and hydroxylamine.
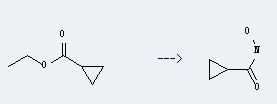
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OCC)C1CC1
(2)InChI: InChI=1/C6H10O2/c1-2-8-6(7)5-3-4-5/h5H,2-4H2,1H3
(3)InChIKey: LDDOSDVZPSGLFZ-UHFFFAOYAD
(4)Std. InChI: InChI=1S/C6H10O2/c1-2-8-6(7)5-3-4-5/h5H,2-4H2,1H3
(5)Std. InChIKey: LDDOSDVZPSGLFZ-UHFFFAOYSA-N
Related Products
- Ethyl (13-cis)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
- ethyl (1R,2R)-1-phenyl-2-(trideuteriomethylamino)cyclohex-3-ene-1-carboxylate,hydrochloride
- Ethyl (1S,2R)-2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate hydrochloride
- Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate
- Ethyl (2-amino-4-hydroxy-6-methyl-5-pyrimidinyl)acetate
- Ethyl (2-bromopropionamido)acetate
- Ethyl (2-cyanoimino-5,6-dichloro-1,2,3,4-tetrahydroquinazolin-3-yl)acetate
- ETHYL (2E,4Z)-DECADIENOATE
- Ethyl (2-hydroxyethyl)dimethyl-ammonium benzilate chloride
- Ethyl (2-mercaptoethyl) carbamate S-ester with O,O-dimethyl phosphorodithioate
- 4606-15-9
- 460-61-7
- 46064-79-3
- 4606-63-7
- 4606-65-9
- 460-73-1
- 460738-38-9
- 460745-20-4
- 460750-26-9
- 460750-27-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View