-
Name
Ezetimibe
- EINECS 682-606-0
- CAS No. 163222-33-1
- Article Data70
- CAS DataBase
- Density 1.334 g/cm3
- Solubility
- Melting Point 164-166 °C
- Formula C24H21F2NO3
- Boiling Point 654.9 °C at 760 mmHg
- Molecular Weight 409.432
- Flash Point 349.9 °C
- Transport Information
- Appearance white powder
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
- Hazard Symbols R36/37/38:;
- Synonyms (-)-Sch 58235;Zetia (TN);Zetia;Ezetimibe 1-(4-flurophenyl)-(3R)-[3-(4-flurophenyl)-(3S)-hydroxypropyl]-(4S)-(4-hydroxyphenyl)-2-azetidinone;2-Azetidinone,1-(4-fluorophenyl)-3-[(3S)-3- (4-fluorophenyl)-3-hydroxypropyl]-4-(4- hydroxyphenyl)-,(3R,4S)-;Sch 58235;Vytorin;Ezedoc;(3R,4S)-1-(4-Fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)-2-azetidinone;
- PSA 60.77000
- LogP 4.95330
Synthetic route
-
-
163222-32-0
(3R,4S)-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4-(4-benzyloxyphenyl)-2-azetidinone

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| With 10 wt% Pd(OH)2 on carbon; hydrogen In methanol; cyclohexane at 70℃; under 760.051 Torr; for 3h; | 99% |
| With 5%-palladium/activated carbon; hydrogen In tetrahydrofuran; ethanol at 50 - 55℃; under 37.5038 - 75.0075 Torr; | 90.3% |
| With palladium 10% on activated carbon; ammonium formate; acetic acid for 6h; Reflux; | 90% |
-
-
873205-85-7
1-(4-fluorophenyl)-3(R)-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4(S)-(4-hydroxyphenyl)-2-azetidinone hydrate

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| In n-heptane; tert-butyl methyl ether at 20℃; Product distribution / selectivity; Heating / reflux; | 99% |
| In Isopropyl acetate at 20℃; Product distribution / selectivity; Heating / reflux; | |
| With acetic acid at 20℃; Product distribution / selectivity; Heating / reflux; |

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| With sulfuric acid In water; isopropyl alcohol at 20 - 25℃; for 1h; | 95% |
| With perchloric acid at 40℃; for 3h; Temperature; Solvent; | 92% |
| With sulfuric acid In water; isopropyl alcohol at 20℃; for 1h; | 91.5% |

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| With pyridine hydrofluoride In tetrahydrofuran; pyridine at 20℃; for 24h; | 93% |
-
-
1232148-26-3
4-((2S,3R)-1-(4-fluorophenyl)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-4-oxoazetidin-2-yl)phenyl pivalate

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| Stage #1: 4-((2S,3R)-1-(4-fluorophenyl)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-4-oxoazetidin-2-yl)phenyl pivalate With sodium hydroxide In tetrahydrofuran; methanol at -15℃; Stage #2: With hydrogenchloride; water In ethyl acetate | 92% |

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| With N,O-bis-(trimethylsilyl)-acetamide; tetrabutyl ammonium fluoride In tert-butyl methyl ether at 20℃; for 0.25h; | 91% |
-
-
191330-56-0
[14C]-Sch 57871

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| With dimethylsulfide borane complex; trifluoroacetic anhydride; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole In dichloromethane; toluene at -25 - -20℃; for 3h; Inert atmosphere; | 90% |
| With glucose dehydrogenase; alpha-D-glucopyranose; NADP; KRED-128 In water; isopropyl alcohol at 35℃; for 1.5h; pH=7; Product distribution / selectivity; Phosphate buffer; | 89.26% |
| With glucose dehydrogenase; potassium phosphate; alpha-D-glucopyranose; DL-dithiothreitol; NADP; magnesium sulfate; KRED-118 In methanol; water at 30℃; pH=7.0; Product distribution / selectivity; | 87.59% |

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| With hydrogen bromide In isopropyl alcohol at 20℃; for 4h; | 87% |
-
-
1004520-48-2
(3R,4S)-1-(4-fluorophenyl)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-4-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)azetidin-2-one

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In acetic acid at 20℃; for 2h; Time; | 86.23% |
| With dihydrogen peroxide In water; isopropyl alcohol at 20℃; for 0.5h; |

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen; acetic acid In ethanol at 25℃; for 4h; pH=3; | 85.4% |

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| With hydrogen fluoride In ethanol at 0 - 10℃; Inert atmosphere; | 80.6% |
-
-
1159183-24-0
benzyl (4-((2S,3R)-1-(4-fluorophenyl)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-4-oxoazetidin-2-yl)phenyl) carbonate

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| With hydrogen; palladium 10% on activated carbon In methanol under 760.051 Torr; for 23h; Product distribution / selectivity; | 76% |

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| With trimethyl(benzyl)ammonium fluoride In propan-1-ol at 20 - 30℃; Inert atmosphere; | 75.1% |

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| Stage #1: C34H35F2NO3Si With sulfuric acid In isopropyl alcohol at 20℃; for 1h; Stage #2: With palladium 10% on activated carbon; hydrogen; ammonium formate In methanol for 2h; Inert atmosphere; | 75% |
-
-
1042722-68-8
C43H35F2NO3

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| With hydrogen; 3% Pd/C In methanol at 20℃; for 18h; Product distribution / selectivity; | 73.3% |
-
-
191330-56-0
[14C]-Sch 57871

-
A
-
163222-33-1
ezetemibe

-
B
-
163380-16-3
(3R,4S)-1-(4-fluorophenyl)-3-[(3R)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl)-azetidin-2-one

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; iodine; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole In tetrahydrofuran; dichloromethane; toluene at -8 - 0℃; for 4h; Corey-Bakshi-Shibata Reduction; Inert atmosphere; stereoselective reaction; | A 55.4% B n/a |
| With hydrogen; palladium 10% on activated carbon In ethanol under 3102.97 Torr; for 16h; | |
| With Rhodococcus fascians MO22 cells at 35℃; pH=7; Microbiological reaction; aq. phosphate buffer; optical yield given as %de; diastereoselective reaction; |
-
-
954109-26-3
(3R,4S)-4-(4-(trimethylsilyloxy)phenyl)-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]azetidin-2-one

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| Stage #1: (3R,4S)-4-(4-(trimethylsilyloxy)phenyl)-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]azetidin-2-one With methanesulfonic acid; dimethylsulfide borane complex; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole In tetrahydrofuran; methanol; toluene at -25 - 25℃; for 2.5 - 2.75h; Stage #2: With hydrogenchloride; water In tetrahydrofuran; methanol; toluene at 0℃; for 0.5h; Product distribution / selectivity; |

| Conditions | Yield |
|---|---|
| With formic acid; triethylamine; (1S,2S)-N-methanesulfonyl-1,2-diphenylethane-1,2-diamine; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2 In tert-butyl methyl ether at 65℃; for 17h; Product distribution / selectivity; | A n/a B n/a |
| With formic acid; triethylamine; dichloro(benzene)ruthenium(II) dimer; (1S,2S)-N-methanesulfonyl-1,2-diphenylethane-1,2-diamine In tert-butyl methyl ether at 65℃; for 17h; Product distribution / selectivity; | A n/a B n/a |
| With formic acid; triethylamine; dichloro(mesitylene)ruthenium(II) dimer; N-((1S,2S)-2-amino-1,2-diphenylethyl)-1,1,1-trifluoromethanesulfonamide In tert-butyl methyl ether at 65℃; for 17h; Product distribution / selectivity; | A n/a B n/a |
-
-
190595-65-4
(3R,4S)-4-[4-(benzyloxy)phenyl]-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]azetidin-2-one

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 70 percent / CBS; BH3-Me2S / toluene; CH2Cl2 / 2 h / -20 °C 2: 79 percent / ammonium formate; HOAc / Pd-C / methanol / 3 h / 45 - 55 °C / pH 3 - 5 View Scheme | |
| Multi-step reaction with 2 steps 1: BH3*Me2S / tetrahydrofuran / 3 h / 22 °C 2: H2 / 10percent Pd/C / ethanol / 16 h / 3102.9 Torr View Scheme | |
| Multi-step reaction with 2 steps 1: dimethylsulfide borane complex; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole / dichloromethane; tetrahydrofuran / 5 h / 0 °C 2: palladium on activated charcoal; hydrogen / methanol / 6 h / 20 °C / 3800.26 Torr View Scheme | |
| Multi-step reaction with 2 steps 1: dimethylsulfide borane complex; (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole / tetrahydrofuran; dichloromethane / 5 h / 0 °C 2: palladium on activated charcoal; hydrogen / methanol / 6 h / 20 °C / 3800.26 Torr View Scheme | |
| Multi-step reaction with 2 steps 1: dimethylsulfide borane complex / tetrahydrofuran / 4 h / -10 °C / Inert atmosphere 2: palladium on activated charcoal; formic acid; ammonium formate / isopropyl alcohol / 3 h / 40 °C View Scheme |
-
-
70627-52-0
4-benzyloxybenzylidene-N-(4-fluoro)phenylanisidine

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 64 percent / LDA; DMPU; LiCl / dimethylformamide; tetrahydrofuran / 18 h / -30 - -25 °C 2: 90 percent / NaIO4 / acetonitrile; H2O / 2 h / 20 °C 3: BF3 etherate / toluene / 0.08 h / -30 °C 4: 99 g / p-toluenesulfonic acid monohydrate; molecular sieves / toluene / 4 h / 40 - 50 °C 5: 90 percent / hydrogen / 10 percent Pd/C / methanol; ethyl acetate / 3 h / 1551.44 Torr 6: 79 percent / bistrimethylsilylurea; BH3-Me2S / CH2Cl2 / 5 h / -20 - -15 °C View Scheme | |
| Multi-step reaction with 7 steps 1: 64 percent / LDA; DMPU; LiCl / dimethylformamide; tetrahydrofuran / 18 h / -30 - -25 °C 2: 90 percent / NaIO4 / acetonitrile; H2O / 2 h / 20 °C 3: BF3 etherate / toluene / 0.08 h / -30 °C 4: 99 g / p-toluenesulfonic acid monohydrate; molecular sieves / toluene / 4 h / 40 - 50 °C 5: 71 percent / (PPh3)3RhCl; hydrogen / CH2Cl2 / 18 h / 3102.89 Torr 6: 70 percent / CBS; BH3-Me2S / toluene; CH2Cl2 / 2 h / -20 °C 7: 79 percent / ammonium formate; HOAc / Pd-C / methanol / 3 h / 45 - 55 °C / pH 3 - 5 View Scheme | |
| Multi-step reaction with 5 steps 1: N-ethyl-N,N-diisopropylamine; titanium(IV) isopropylate; titanium tetrachloride / dichloromethane / 3 h / -5 °C 2: N,O-Bis(trimethylsilyl)acetamide; tetrabutyl ammonium fluoride / toluene / 48 h / Reflux 3: formic acid / dichloromethane / 12 h / Reflux 4: dimethyl sulfide borane / dichloromethane; toluene; tetrahydrofuran / 12 h / 15 °C 5: acetic acid; ammonium formate; palladium 10% on activated carbon / 6 h / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: N-ethyl-N,N-diisopropylamine; titanium(IV) isopropylate; titanium tetrachloride / dichloromethane / 5 h / -30 - -20 °C 2.1: tetrabutyl ammonium fluoride / toluene / 3 h / 60 °C 3.1: sulfuric acid / isopropyl alcohol / 1 h / 20 °C 3.2: 2 h / Inert atmosphere View Scheme | |
| Multi-step reaction with 3 steps 1: N,O-bis-(trimethylsilyl)-acetamide; tetrabutyl ammonium fluoride / toluene / 60 °C 2: dimethylsulfide borane complex / dichloromethane; toluene / 3 h / -5 - 0 °C / Inert atmosphere 3: palladium 10% on activated carbon; hydrogen / methanol; tetrahydrofuran / 2 h View Scheme |
-
-
221349-58-2
(2S,3S)-2-(4-(benzyloxy)phenyl)-1-(4-fluorophenyl)-4-oxazetidine-3-carbaldehyde

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: BF3 etherate / toluene / 0.08 h / -30 °C 2: 99 g / p-toluenesulfonic acid monohydrate; molecular sieves / toluene / 4 h / 40 - 50 °C 3: 90 percent / hydrogen / 10 percent Pd/C / methanol; ethyl acetate / 3 h / 1551.44 Torr 4: 79 percent / bistrimethylsilylurea; BH3-Me2S / CH2Cl2 / 5 h / -20 - -15 °C View Scheme | |
| Multi-step reaction with 5 steps 1: BF3 etherate / toluene / 0.08 h / -30 °C 2: 99 g / p-toluenesulfonic acid monohydrate; molecular sieves / toluene / 4 h / 40 - 50 °C 3: 71 percent / (PPh3)3RhCl; hydrogen / CH2Cl2 / 18 h / 3102.89 Torr 4: 70 percent / CBS; BH3-Me2S / toluene; CH2Cl2 / 2 h / -20 °C 5: 79 percent / ammonium formate; HOAc / Pd-C / methanol / 3 h / 45 - 55 °C / pH 3 - 5 View Scheme |
-
-
221349-56-0
(3S,4S)-4-(4-Benzyloxy-phenyl)-3-((S)-1,2-dihydroxy-ethyl)-1-(4-fluoro-phenyl)-azetidin-2-one

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 90 percent / NaIO4 / acetonitrile; H2O / 2 h / 20 °C 2: BF3 etherate / toluene / 0.08 h / -30 °C 3: 99 g / p-toluenesulfonic acid monohydrate; molecular sieves / toluene / 4 h / 40 - 50 °C 4: 90 percent / hydrogen / 10 percent Pd/C / methanol; ethyl acetate / 3 h / 1551.44 Torr 5: 79 percent / bistrimethylsilylurea; BH3-Me2S / CH2Cl2 / 5 h / -20 - -15 °C View Scheme | |
| Multi-step reaction with 6 steps 1: 90 percent / NaIO4 / acetonitrile; H2O / 2 h / 20 °C 2: BF3 etherate / toluene / 0.08 h / -30 °C 3: 99 g / p-toluenesulfonic acid monohydrate; molecular sieves / toluene / 4 h / 40 - 50 °C 4: 71 percent / (PPh3)3RhCl; hydrogen / CH2Cl2 / 18 h / 3102.89 Torr 5: 70 percent / CBS; BH3-Me2S / toluene; CH2Cl2 / 2 h / -20 °C 6: 79 percent / ammonium formate; HOAc / Pd-C / methanol / 3 h / 45 - 55 °C / pH 3 - 5 View Scheme |

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: BF3 etherate / toluene / 0.08 h / -30 °C 2: 99 g / p-toluenesulfonic acid monohydrate; molecular sieves / toluene / 4 h / 40 - 50 °C 3: 90 percent / hydrogen / 10 percent Pd/C / methanol; ethyl acetate / 3 h / 1551.44 Torr 4: 79 percent / bistrimethylsilylurea; BH3-Me2S / CH2Cl2 / 5 h / -20 - -15 °C View Scheme | |
| Multi-step reaction with 5 steps 1: BF3 etherate / toluene / 0.08 h / -30 °C 2: 99 g / p-toluenesulfonic acid monohydrate; molecular sieves / toluene / 4 h / 40 - 50 °C 3: 71 percent / (PPh3)3RhCl; hydrogen / CH2Cl2 / 18 h / 3102.89 Torr 4: 70 percent / CBS; BH3-Me2S / toluene; CH2Cl2 / 2 h / -20 °C 5: 79 percent / ammonium formate; HOAc / Pd-C / methanol / 3 h / 45 - 55 °C / pH 3 - 5 View Scheme |
-
-
221349-60-6
(3R,4S)-4-(4-Benzyloxy-phenyl)-1-(4-fluoro-phenyl)-3-[(E)-3-(4-fluoro-phenyl)-3-oxo-propenyl]-azetidin-2-one

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 90 percent / hydrogen / 10 percent Pd/C / methanol; ethyl acetate / 3 h / 1551.44 Torr 2: 79 percent / bistrimethylsilylurea; BH3-Me2S / CH2Cl2 / 5 h / -20 - -15 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 71 percent / (PPh3)3RhCl; hydrogen / CH2Cl2 / 18 h / 3102.89 Torr 2: 70 percent / CBS; BH3-Me2S / toluene; CH2Cl2 / 2 h / -20 °C 3: 79 percent / ammonium formate; HOAc / Pd-C / methanol / 3 h / 45 - 55 °C / pH 3 - 5 View Scheme |
-
-
231301-00-1
(3S,4S)-4-(4-Benzyloxy-phenyl)-1-(4-fluoro-phenyl)-3-[3-(4-fluoro-phenyl)-1-hydroxy-3-oxo-propyl]-azetidin-2-one

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 99 g / p-toluenesulfonic acid monohydrate; molecular sieves / toluene / 4 h / 40 - 50 °C 2: 90 percent / hydrogen / 10 percent Pd/C / methanol; ethyl acetate / 3 h / 1551.44 Torr 3: 79 percent / bistrimethylsilylurea; BH3-Me2S / CH2Cl2 / 5 h / -20 - -15 °C View Scheme | |
| Multi-step reaction with 4 steps 1: 99 g / p-toluenesulfonic acid monohydrate; molecular sieves / toluene / 4 h / 40 - 50 °C 2: 71 percent / (PPh3)3RhCl; hydrogen / CH2Cl2 / 18 h / 3102.89 Torr 3: 70 percent / CBS; BH3-Me2S / toluene; CH2Cl2 / 2 h / -20 °C 4: 79 percent / ammonium formate; HOAc / Pd-C / methanol / 3 h / 45 - 55 °C / pH 3 - 5 View Scheme |
-
-
219653-96-0
(Z)-1-(4-(benzyloxy)phenyl)-N-(4-fluorophenyl)methaneimine

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: (nBu)3N / heptane; toluene / 80 - 90 °C 2: LiOH*H2O / methanol / 2 h 3: oxalyl chloride / CH2Cl2 / 16 h / 22 °C 4: (Ph3P)4Pd / tetrahydrofuran / 1 h / 10 °C 5: BH3*Me2S / tetrahydrofuran / 3 h / 22 °C 6: H2 / 10percent Pd/C / ethanol / 16 h / 3102.9 Torr View Scheme |
-
-
204589-82-2
(3R,4S)-1-(4-fluorophenyl)-3-(3-hydroxy-3-oxopropyl)-4-(4-benzyloxyphenyl)-2-azetidinone

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: oxalyl chloride / CH2Cl2 / 16 h / 22 °C 2: (Ph3P)4Pd / tetrahydrofuran / 1 h / 10 °C 3: BH3*Me2S / tetrahydrofuran / 3 h / 22 °C 4: H2 / 10percent Pd/C / ethanol / 16 h / 3102.9 Torr View Scheme |
-
-
204589-84-4
3-((3R,4S)-1-(4-fluorophenyl)-2-oxo-4-(4-(benzyloxy)phenyl)azetidin-3-yl)propionic acid chloride

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: (Ph3P)4Pd / tetrahydrofuran / 1 h / 10 °C 2: BH3*Me2S / tetrahydrofuran / 3 h / 22 °C 3: H2 / 10percent Pd/C / ethanol / 16 h / 3102.9 Torr View Scheme |
-
-
204589-80-0
methyl 3-((2S,3R)-2-(4-(benzyloxy)phenyl)-1-(4-fluorophenyl)-4-oxoazetidin-3-yl)propanoate

-
-
163222-33-1
ezetemibe

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: LiOH*H2O / methanol / 2 h 2: oxalyl chloride / CH2Cl2 / 16 h / 22 °C 3: (Ph3P)4Pd / tetrahydrofuran / 1 h / 10 °C 4: BH3*Me2S / tetrahydrofuran / 3 h / 22 °C 5: H2 / 10percent Pd/C / ethanol / 16 h / 3102.9 Torr View Scheme | |
| Multi-step reaction with 4 steps 1.1: diethyl aluminiumcholoride / toluene / -12 - 20 °C / Inert atmosphere 2.1: magnesium / tetrahydrofuran / 1 h / 40 - 50 °C / Inert atmosphere 2.2: 1 h / -5 - 0 °C 3.1: [(S,S)-teth-TsDpen RuCl]; triethylamine; formic acid / ethylbenzene / 24 h / 35 - 40 °C / Inert atmosphere 4.1: 5%-palladium/activated carbon; hydrogen / tetrahydrofuran; ethanol / 50 - 55 °C / 37.5 - 75.01 Torr View Scheme |
-
-
108-24-7
acetic anhydride

-
-
163222-33-1
ezetemibe

-
-
163380-20-9
4(S)-(4-acetyloxyphenyl)-3(R)-(3(S)-acetyloxy-3-(4-fluorophenyl)propyl)-1-(4-fluorophenyl)-2-azetidinone

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane | 100% |
| With dmap; triethylamine In dichloromethane at 20℃; for 2h; | 96% |
-
-
456-64-4
phenyl trifluoromethanesulfonamide

-
-
163222-33-1
ezetemibe

-
-
847781-45-7
(trifluoromethanesulfonyl)ezetimibe

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 20℃; for 3.5h; | 99% |
| With dmap; triethylamine In dichloromethane at 20℃; for 3.5h; | 96% |
-
-
100-39-0
benzyl bromide

-
-
163222-33-1
ezetemibe

-
-
163222-32-0
(3R,4S)-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3(S)-hydroxypropyl]-4-(4-benzyloxyphenyl)-2-azetidinone

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 20℃; for 3h; | 99% |
-
-
108-24-7
acetic anhydride

-
-
163222-33-1
ezetemibe

-
-
795306-53-5
Acetic acid 4-{(2S,3R)-1-(4-fluoro-phenyl)-3-[(S)-3-(4-fluoro-phenyl)-3-hydroxy-propyl]-4-oxo-azetidin-2-yl}-phenyl ester

| Conditions | Yield |
|---|---|
| With sodium hydroxide In isopropyl alcohol for 5h; | 97% |
| With sodium hydroxide In water; isopropyl alcohol for 5h; | 97% |
-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
163222-33-1
ezetemibe

-
-
849799-39-9
3-[3-(tert-butyl-dimethyl-silanyloxy)-3-(4-fluoro-phenyl)-propyl]-4-[4-(tert-butyl-dimethyl-silanyloxy)-phenyl]-1-(4-fluoro-phenyl)-azetidin-2-one

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide for 5h; | 97% |
| With 1H-imidazole In DMF (N,N-dimethyl-formamide) for 5h; | 97% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
163222-33-1
ezetemibe

-
-
847781-45-7
(trifluoromethanesulfonyl)ezetimibe

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 20℃; for 3.5h; | 96% |
| With dmap; triethylamine In dichloromethane at 23℃; for 3h; | 95% |
| With dmap; triethylamine In dichloromethane at 23℃; for 3h; | 95% |
| With dmap; triethylamine In dichloromethane at 20℃; for 3h; Inert atmosphere; | 562.7 mg |
-
-
501-53-1
benzyl chloroformate

-
-
163222-33-1
ezetemibe

-
-
1159183-24-0
benzyl (4-((2S,3R)-1-(4-fluorophenyl)-3-((S)-3-(4-fluorophenyl)-3-hydroxypropyl)-4-oxoazetidin-2-yl)phenyl) carbonate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 0.5h; | 92% |
Ezetimibe Chemical Properties
Name: Ezetimibe
Synonyms: 1-(4-Fluorophenyl)-3-[3-(4-fluorophenyl)-3-hydroxy-propyl]-4-(4-hydroxyphenyl)-azetidin-2-one
CAS Registry Number: 163222-33-1
Molecular Formula: C24H21F2NO3
Molecular Weight: 409.43
Molecular Structure: 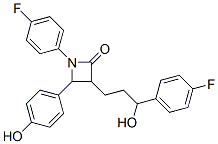
Mp: 164-166°C
Ezetimibe Uses
Ezetimibe is an anti-hyperlipidemic medication which is used to lower Cholesterol levels. It acts by decreasing Cholesterol absorption in the intestine. It could be used alone when other Cholesterol-lowering medications are not tolerated, or when Cholesterol levels are unable to be controlled on statins alone, ezetimibe could be used together with statins
Ezetimibe Safety Profile
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
Use cautiously in:
1)breastfeeding patients
2)children younger than age 10
3)pregnant patients not receiving HMG-CoA reductase inhibitors
4)renal or hepatic impairment
5)elderly patients.
Ezetimibe Specification
Patient monitoring:
1)Monitor hepatic and lipid profiles
2)Assess for and report unexplained muscle pain.
Related Products
- Ezetimibe
- 163229-48-9
- 16323-43-6
- 16324-27-9
- 163252-36-6
- 16325-23-8
- 163253-35-8
- 16325-88-5
- 163260-73-9
- 163260-77-3
- 163266-17-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View